Applications of Exome Sequencing in Animals
The rapid advancement of genomic sequencing techniques has significantly improved our ability to investigate the genetic basis of diverse biological phenomena. Exome sequencing, in particular, has emerged as a powerful tool in animal genetics research. By targeting protein-coding regions-key segments of DNA that govern phenotypic traits-this approach enables precise examination of functionally relevant genetic variations. This review discusses the wide-ranging applications of exome sequencing in animal science, emphasizing its role in uncovering disease-linked genes, enhancing selective breeding programs, and elucidating evolutionary processes.
What is Exome Sequencing
Exome sequencing is a targeted genomic technique that selectively analyzes protein-coding regions of the genome. Unlike whole-genome sequencing, which assesses the entire DNA sequence, this approach focuses exclusively on exons-the segments that dictate protein synthesis and influence phenotypic traits. Leveraging high-throughput sequencing, it efficiently captures and examines these functionally critical regions, enabling precise detection of genetic variants.
In animal studies, exome sequencing offers distinct advantages. Its targeted design reduces costs compared to whole-genome sequencing while maintaining high biological relevance. Additionally, it provides exceptional accuracy in identifying coding-region mutations, including SNPs and small InDels. The method's versatility extends to both model and non-model species, making it invaluable for functional genomics, comparative studies, and evolutionary biology.
Technical Principles of Exome Sequencing
Exome sequencing is a targeted approach that focuses on sequencing the protein-coding regions of the genome, known as exons. These exons, which comprise only 1% to 2% of the entire genome, contain most disease-related genetic variants.
1) Sample Preparation
High-quality genomic DNA is extracted from biological samples. The quality and quantity of the DNA are assessed to ensure it is suitable for sequencing.
2) Library Preparation
The extracted DNA is fragmented into smaller pieces using methods such as sonication or enzymatic digestion. Adapters are then ligated to the fragmented DNA ends to facilitate binding to the sequencing platform.
3) Exome Enrichment
The exonic regions are selectively captured using various methods. The most common approach is aqueous-phase hybridization capture, where biotinylated probes are used to hybridize with the exons. Magnetic streptavidin beads bind to the biotinylated probes, allowing the capture of exonic fragments. Non-target regions are washed away, and the captured exonic fragments are amplified.
4) Sequencing
The enriched exome library is sequenced using high-throughput sequencing platforms. This process generates millions of short overlapping reads, which are then aligned to the reference genome. Paired-end sequencing, which sequences both the forward and reverse strands, improves alignment accuracy.
5) Data Analysis
The raw sequencing data are pre-processed and aligned to the reference genome, and PCR duplicates are removed. Computational tools are used to identify genetic variants, such as single nucleotide variants (SNVs) and insertions/deletions (InDels). These variants are annotated to identify potentially pathogenic mutations.
Factors Affecting Exome Sequencing
Several factors influence the efficiency and accuracy of exome sequencing:
1) Exon Size and GC Content: These factors affect the design of capture probes and the uniformity of coverage.
2) Repeat Elements and Segmental Duplications: These genomic features can complicate probe design and sequencing alignment.
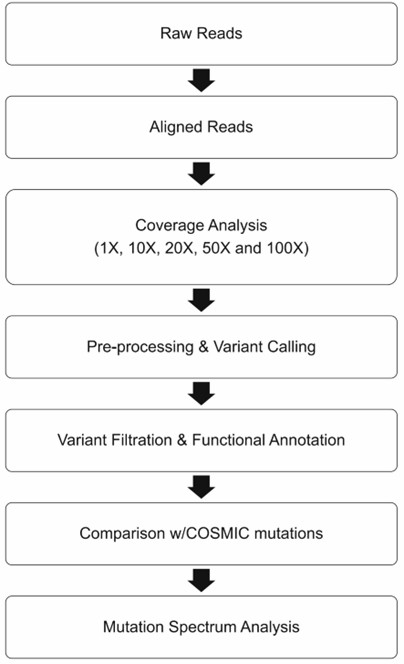 Figure 1. Bioinformatic evaluation pipeline for variant detection. I.( Julie F. Foley .2018)
Figure 1. Bioinformatic evaluation pipeline for variant detection. I.( Julie F. Foley .2018)
Services you may interested in
Learn More
Applications of Exome Sequencing in Animal Disease Research
Disease Gene Identification
Exome sequencing has been instrumental in identifying genes associated with various diseases in animals. For example, in dogs, exome sequencing has been used to identify causal mutations for several Mendelian disorders, such as progressive retinal atrophy, nemaline myopathy, and neuroaxonal dystrophy. In a recent study, exome sequencing was used to identify a frameshift mutation in the CNGB1 gene associated with progressive retinal atrophy in Papillon and Phalène dogs. Similarly, a missense mutation in the PLA2G6 gene was identified in dogs with neuroaxonal dystrophy using exome sequencing.
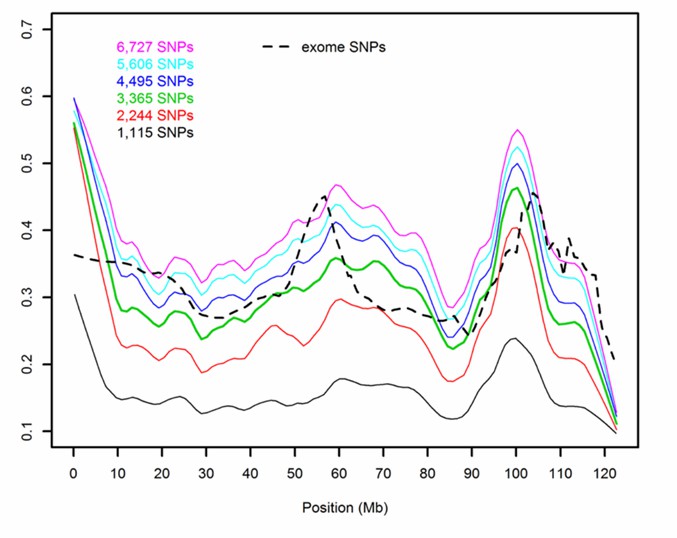 Figure 2. Analysis of exome SNPs in dogs.( Bart J. G .2017)
Figure 2. Analysis of exome SNPs in dogs.( Bart J. G .2017)
Understanding Disease Mechanisms
Exome sequencing plays a vital role in uncovering the molecular basis of diseases in animals. By examining how specific genetic variations influence protein structure and function, researchers can gain insights into the biological pathways involved in the development and progression of various conditions. For instance, in investigations of neurodegenerative disorders using animal models, this technique has been instrumental in identifying mutations that impair neuronal activity and trigger disease manifestation. Such insights are fundamental to the design of precise therapeutic strategies and targeted medical interventions.
Disease Model Construction and Validation
Exome sequencing also plays a key role in the development and validation of animal models for human diseases. By introducing specific genetic mutations uncovered through exome analysis, scientists can engineer animal models that closely replicate human pathological conditions. These models are essential for exploring disease mechanisms, evaluating potential treatments, and deepening our understanding of disease biology. For example, in cancer studies, exome sequencing has been utilized to pinpoint tumor-related mutations, which are then replicated in animal systems to investigate tumor formation and assess anti-cancer drug efficacy.
Rats, due to their genetic and physiological resemblance to humans, have long been a preferred model in biomedical research. The advancement of whole exome sequencing (WES) technologies in rats has paved the way for more precise identification of disease-associated mutations. By analyzing mutations in cancer-related genes from chemically induced rat tumor cell lines and comparing them with validated cancer-associated genes in the COSMIC (Catalogue of Somatic Mutations in Cancer) database, researchers can better understand the genetic landscape of cancer and uncover novel targets for therapeutic intervention.
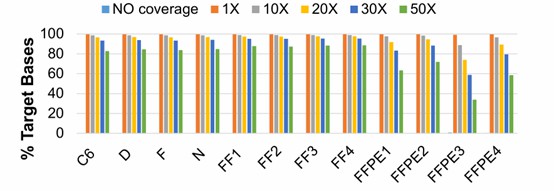 Figure 3. Breadth of reference genome coverage.( Julie F. Foley .2018)
Figure 3. Breadth of reference genome coverage.( Julie F. Foley .2018)
Applications in Animal Genetic Breeding
Identification of Genes for Desirable Traits
Exome sequencing is a powerful tool for identifying genes associated with desirable traits in animals. For example, exome sequencing has been used in livestock and poultry breeding to locate genes that influence growth rate, meat quality, milk production, and other economically important traits. Researchers can identify specific genetic variants that contribute to these traits by analyzing the exome sequences of animals with superior phenotypes. This information can then be used to guide selective breeding programs, accelerating the development of improved animal varieties.
 Figure 4. Strategies for finding disease-causing rare variants using exome sequencing.( Bamshad, M., 2011)
Figure 4. Strategies for finding disease-causing rare variants using exome sequencing.( Bamshad, M., 2011)
Assessment of Genetic Diversity
Exome sequencing also enables the assessment of genetic diversity within animal populations. By analyzing the variation in exonic regions, researchers can gain insights into the genetic structure of populations, identify inbreeding issues, and detect potential genetic bottlenecks. This information is particularly valuable for the conservation of endangered species and the optimization of breeding programs. For example, in conserving wild animal populations, exome sequencing can help identify individuals with unique genetic variants crucial for maintaining genetic diversity and population viability.
Molecular Breeding and Marker-Assisted Selection
Exome sequencing data can be utilized in molecular breeding and marker-assisted selection (MAS) strategies. By identifying genetic markers linked to desirable traits, breeders can select individuals carrying these markers for inclusion in breeding programs. This approach significantly speeds up the breeding process and increases the efficiency of producing animals with desired characteristics. For instance, in aquaculture, exome sequencing has been used to identify genes associated with disease resistance, enabling the development of more resilient fish and shrimp varieties through MAS.
Applications in Animal Evolutionary Biology
Species Evolution Studies
Exome sequencing has become an essential tool in the study of animal species evolution. By comparing the exome sequences of different species, researchers can reconstruct phylogenetic relationships and gain insights into the evolutionary history of animals. For example, exome sequencing has helped elucidate the genetic differences between humans and other primates in the study of primate evolution, providing valuable information on the molecular basis of human-specific traits. This comparative approach allows researchers to trace the evolutionary trajectories of various species and understand the genetic changes that have driven their diversification.
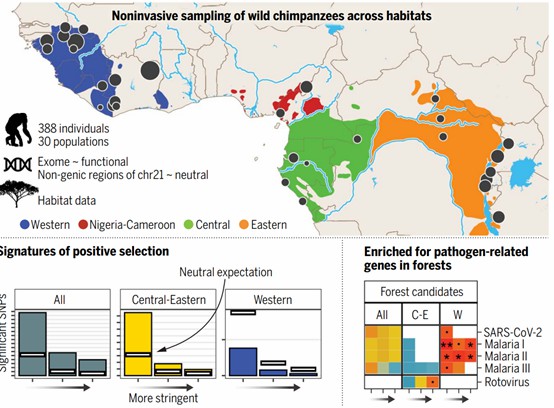 Figure 5. Chimpanzee exome dataset distribution, sample size, and coverage.( Bamshad, M., 2011)
Figure 5. Chimpanzee exome dataset distribution, sample size, and coverage.( Bamshad, M., 2011)
Analysis of Adaptive Evolution
Exome sequencing also facilitates the analysis of adaptive evolution in animals. By examining the genetic variations in exonic regions of animals living in different environments, researchers can identify genes that have undergone positive selection and contributed to adaptive traits. For instance, in high-altitude animals such as yaks, exome sequencing has been used to study the genetic adaptations that enable these animals to thrive in low-oxygen environments. Understanding the molecular mechanisms of adaptive evolution is crucial for comprehending how animals have evolved to survive in diverse ecological niches.
Challenges and Future Directions
Technical Challenges
Despite its many advantages, exome sequencing faces several technical challenges. These include the efficiency of exon capture, the accuracy of variant detection, and the complexity of data analysis. The incomplete coverage of non-coding regions, which may also harbor important regulatory elements, is another limitation of exome sequencing. Additionally, the interpretation of genetic variants in the context of complex phenotypes remains a significant challenge, requiring advanced bioinformatics tools and expertise.
Future Developments
To overcome these challenges, future developments in exome sequencing technology are expected to focus on improving capture efficiency, reducing costs, and enhancing the accuracy of variant detection. The integration of exome sequencing with other omics technologies, such as single-cell sequencing and epigenetics, will provide a more comprehensive understanding of the genetic and epigenetic factors underlying animal traits and diseases. Furthermore, the development of more sophisticated bioinformatics pipelines and databases will facilitate the analysis and interpretation of exome sequencing data, making it more accessible to researchers in various fields.
Conclusion
Exome sequencing has emerged as a powerful tool in animal research, offering significant advantages in various fields such as disease research, genetic breeding, and evolutionary biology. By focusing on the protein-coding regions of the genome, exome sequencing efficiently captures the genetic variants that are most likely to impact phenotypes and diseases.
This targeted approach has greatly enhanced our understanding of the genetic basis of animal traits, enabling researchers to identify specific genes and mutations associated with various conditions. In disease research, exome sequencing has facilitated the discovery of causative mutations for inherited disorders, providing critical insights for developing diagnostic tools and therapeutic strategies. In genetic breeding, it has enabled the selection of desirable traits and the improvement of livestock and companion animals. Additionally, exome sequencing has shed light on evolutionary processes by comparing the coding regions across different species. As the technology continues to advance, becoming more accurate and cost-effective, exome sequencing is set to play an even more pivotal role in driving the future of animal science, promising new breakthroughs and applications.
References:
- Broeckx, B.J.G., Derrien, T., Mottier, S. et al. An exome sequencing based approach for genome-wide association studies in the dog. Sci Rep 7, 15680 (2017). https://doi.org/10.1038/s41598-017-15947-9
- Foley, J. F., Phadke, D. P., Hardy, O., Hardy, S., Miller, V., Madan, A., Howard, K., Kruse, K., Lord, C., Ramaiahgari, S., Solomon, G. G., Shah, R. R., Pandiri, A. R., Herbert, R. A., Sills, R. C., & Merrick, B. A. (2018). Whole exome sequencing in the rat. BMC genomics, 19(1), 487. https://doi.org/10.1186/s12864-018-4858-8
- Bamshad, M., Ng, S., Bigham, A. et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet 12, 745–755 (2011). https://doi.org/10.1038/nrg3031
- Ostridge, H. J., Fontsere, C., et, al. (2024). Local genetic adaptation to habitat in wild chimpanzees. science, 2024.07.09.601734. https://doi.org/10.1101/2024.07.09.601734


 Sample Submission Guidelines
Sample Submission Guidelines
