Gene expression stands as a cornerstone of molecular biology, linking the static genetic code within DNA to the dynamic traits observed in cells and organisms. Through a tightly regulated cascade of molecular events, cells interpret genomic instructions to produce functional outputs—primarily proteins—that determine cellular identity, function, and interaction with the environment. This review explores the fundamental principles, regulatory mechanisms, expression modalities, research methodologies, and genomic frameworks that underpin current understandings of gene expression.
The Central Dogma: Foundational Principles of Gene Expression
Gene expression is the biological process by which information stored in DNA is transcribed into RNA and then translated into functional molecules such as proteins. This pathway is central to the function of all living systems and underpins processes ranging from embryonic development to environmental adaptation.
Definition and Biological Relevance
Gene expression refers to the temporal and spatial process by which a gene's information is converted into RNA or protein products. It is a regulated process essential for:
- Realizing genetic information functionally,
- Driving cellular differentiation and identity,
- Enabling organismal responses to environmental cues.
The Central Dogma Pathway: DNA → RNA → Protein
The classical framework of molecular biology, known as the central dogma, illustrates the directional flow of genetic information:
Transcription: In eukaryotic cells, the process begins within the nucleus, where specific regions of DNA—genes—are transcribed into RNA by the enzyme RNA polymerase II. This process involves the recognition of promoter sequences upstream of coding regions, followed by the synthesis of a complementary RNA strand, known initially as pre-mRNA. This primary transcript undergoes extensive post-transcriptional modifications, including 5′ capping (to protect RNA and assist in ribosome binding), splicing (to remove non-coding introns and ligate exons), and 3′ polyadenylation (to enhance mRNA stability and export). The resulting mature mRNA is a linear, translatable transcript ready for protein synthesis.
Translation: Once processed, the mature mRNA is transported from the nucleus to the cytoplasm, where it associates with ribosomes, the molecular machines of protein synthesis. The ribosome reads the nucleotide sequence of the mRNA in codons—triplets of bases—each specifying a particular amino acid. With the aid of transfer RNAs (tRNAs) and translation factors, amino acids are sequentially assembled into a polypeptide chain. This chain then undergoes folding—often aided by molecular chaperones—and post-translational modifications to form a functional protein capable of performing diverse cellular roles, from catalyzing biochemical reactions to mediating signal transduction and maintaining structural integrity.
 Figure 1. The central dogma pathway.(Wong, F. et al. 2022)
Figure 1. The central dogma pathway.(Wong, F. et al. 2022)
Molecular Architecture: DNA and Genes
DNA, a double-helix polymer composed of nucleotides, stores the blueprint of life. Within it, genes serve as functional units that code for proteins or regulatory RNAs. Despite comprising only ~2% of the genome, protein-coding genes are pivotal in maintaining life. These gene segments are inheritable, and their expression determines an organism’s phenotype—observable traits or behaviors.
Initiation of Expression: Signal Reception and RNA Polymerase Activation
Gene expression often begins with extracellular signals, such as hormones or ligands, interacting with membrane proteins. These receptor proteins initiate intracellular signaling cascades that ultimately activate RNA polymerase. This enzyme binds to gene promoter regions and catalyzes transcription, reading DNA to produce mRNA templates for protein synthesis.
Multi-Tiered Regulation of Gene Expression
Gene expression is regulated through a complex, multi-layered system to ensure proper temporal, spatial, and quantitative control.
Definition and Regulatory Scope
Regulation of gene expression encompasses the mechanisms by which genes are selectively activated or repressed, tailored to developmental stages, cell types, and environmental contexts. Gene products may be proteins or non-coding RNAs (e.g., rRNA, tRNA, miRNA), each contributing uniquely to cellular physiology:
A. DNA-Level Regulation
- Structural Alterations
Gene activation or repression may result from DNA rearrangement or gene amplification.
CpG island methylation at promoter regions often compacts chromatin and suppresses transcription.
- Epigenetic Control
Epigenetic mechanisms—heritable but sequence-independent modifications—play key roles in development, disease, and cellular memory.
B. Transcriptional Control (Primary Regulatory Level)
- Cis-Regulatory Elements
DNA sequences such as "promoters", enhancers, silencers, and insulators dictate transcription initiation and strength.
- Trans-Acting Regulators
Transcription factors bind cis-elements to regulate transcription.
General transcription factors support basal transcription.
Specific transcription factors fine-tune expression in response to signals.
- Chromatin Dynamics
Histone acetylation loosens chromatin to promote transcription.
Histone methylation may activate or silence transcription, depending on residue context.
C. Post-Transcriptional Regulation
- Alternative Splicing
A single gene can generate multiple mRNA isoforms by selectively splicing exons, expanding protein diversity.
- mRNA Stability and Translational Readiness
Features like the 5′cap and poly(A) tail stabilize transcripts and enhance translation efficiency.
D. ranslational and Post-Translational Controls
- Translational Initiation Regulation
Phosphorylation of eIF-2α inhibits translation initiation during stress responses.
- RNA Silencing
miRNAs and siRNAs target mRNAs for degradation or translational repression.
- Post-Translational Modifications
Protein activity, localization, and stability are modulated by modifications such as phosphorylation, ubiquitination, and glycosylation.
 Figure 2. Gene regulation in eukaryotes.( Falk Wachowius et al. 2017)
Figure 2. Gene regulation in eukaryotes.( Falk Wachowius et al. 2017)
Unique Features of Eukaryotic Gene Regulation
- Eukaryotic genes typically include numerous introns and non-coding regions.
- Chromatin structure is a key regulatory determinant.
- Expression is highly spatiotemporally controlled, allowing responsiveness to diverse cues.
Constitutive vs. Inducible Gene Expression
Gene expression patterns are broadly classified as constitutive or inducible, reflecting differences in regulatory responsiveness and functional roles.
Constitutive Expression
Definition: Genes expressed continuously at constant levels, irrespective of environmental conditions.
Function: Typically encode proteins essential for fundamental cellular activities.
Characteristics:
- Persistent expression ("always on"),
- Stable RNA/protein levels,
- Unaffected by signaling inputs.
Inducible Expression
Definition: Genes activated only under specific physiological or environmental conditions.
Function: Mediate adaptive functions, such as stress responses or metabolic shifts.
Characteristics:
- Triggered by inducers (e.g., substrates, hormones),
- Expression varies with environmental changes,
- Regulated by activators or repressors.
Comparison Summary
Expression Stability: Constitutive gene expression is of constant rate, and its expression rate remains stable regardless of changes in the environment of the cell. While inducible gene expression only occurs under specific conditions, its expression rate will change with the variation of the inducing conditions.
Gene Activation State: Constitutive genes generally remain in an activated state and continuously express their products. While inducible genes only activate when needed, they are in a deactivated state otherwise and will only start to express upon receiving a specific inducing signal.
Investigative Strategies in Gene Expression Research
Deciphering gene expression requires both computational and experimental approaches to probe gene function and regulatory complexity.
Functional Genomic Approaches
In Silico Predictions: Utilize databases and algorithms to predict gene functions and interactions.
Experimental Validation: Confirms predicted functions through laboratory assays.
Bioinformatics Resources
NCBI Gene: Centralized resource for gene sequences, structures, and expression data.
Gene Ontology (GO): Classifies genes by biological process, molecular function, and cellular component.
KEGG and KOBAS: Link genes to metabolic and signaling pathways..
Expression Profiling
Quantitative Analyses: Tools such as RT-qPCR, Western blotting, and Northern blotting quantify gene and protein expression.
Spatial Localization: Methods like FISH, GFP tagging, and cell fractionation determine expression sites.
Transcript Variants: Techniques such as 5′/3′ RACE identify alternative splicing products..
Functional Characterization
In Vitro Methods: Include overexpression systems, RNAi, and CRISPR-based gene editing.
In Vivo Models: Transgenic or knockout organisms used to assess physiological roles.
Rescue Experiments: Reintroduction of gene function to validate causality.
Mechanistic Dissection
Interaction Mapping: Techniques like Yeast Two-Hybrid, Co-IP, ChIP, and RIP reveal interacting molecules.
Pathway Elucidation: Multi-omics integration helps chart regulatory and signaling networks.
Functional Genomics: Bridging Sequence and Phenotype
Functional genomics aims to decode how genetic information translates into phenotypic traits via large-scale, systems-level analyses.
Historical Context and Definitions
Coined in 1986, "functional genomics" emerged to define the shift from genome sequencing to functional annotation, especially post-Human Genome Project.
Core Research Domains
Gene Function Analysis
Explores gene roles in cellular and developmental contexts.
Differential Expression Studies
Compare gene activity across tissues, stages, or conditions to identify regulatory patterns.
Proteomic Profiling
Proteomics studies the full set of proteins encoded by the genome and their functional states.
Genomic Variation
Investigates how sequence variation drives phenotypic diversity and evolutionary change.
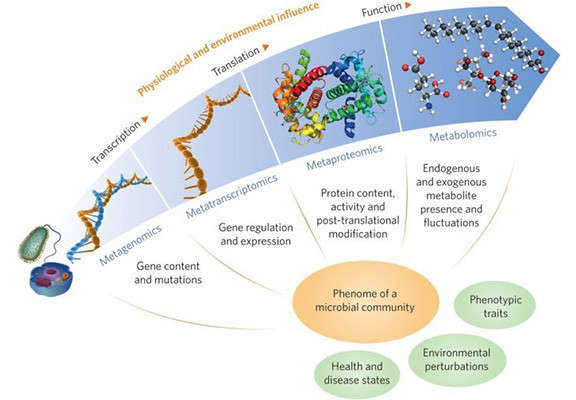
 Figure 3. Genomics research fields spanning multiple biological levels.( Goh, H. H. et al. 2018)
Figure 3. Genomics research fields spanning multiple biological levels.( Goh, H. H. et al. 2018)
Technological Platforms
Transcriptomics
Techniques like SAGE, microarrays, and RNA-seq detect gene expression patterns.
Proteomics
2D gel electrophoresis and mass spectrometry facilitate protein identification and quantification.
Genetic Manipulation
Approaches like gene knockouts, RNAi, and transgenics experimentally test gene function.
Computational Tools
Bioinformatics integrates data from various omics layers to model pathways and predict phenotypic outcomes.
Linking Genotype to Phenotype
Genotype: The genetic constitution of an organism.
Phenotype: The resultant traits shaped by gene expression and environmental interactions.
Although foundational theories (e.g., Johannsen, 1908) initiated this field, complex phenotypes—especially in monogenic disorders—continue to challenge prediction models. Multi-dimensional analyses are essential to resolve these complexities.
Future Perspectives and Research Frontiers
Despite major progress, key challenges remain:
Predictive Limitations: Accurately linking genotype to complex traits is still elusive.
Technological Advancements: More sensitive, high-resolution omics tools are necessary.
Application Gaps: Bridging foundational research with clinical and industrial applications remains a priority.
Emerging approaches—multi-omics integration, AI-driven analytics, and synthetic biology—promise to redefine our capacity to interpret and manipulate gene expression for scientific and practical benefit.
Conclusion
Gene expression is a multi-dimensional, tightly regulated process that translates genetic information into biological function. Through layers of transcriptional, post-transcriptional, translational, and epigenetic control, cells fine-tune gene activity to develop, specialize, and adapt. Ongoing advances in genomics, systems biology, and molecular technologies are continuously unveiling the complexity of gene regulation, drawing us closer to fully decoding the biological language embedded within the genome.
References
- Diercks, C. S., Dik, D. A., & Schultz, P. G. (2021). Adding New Chemistries to the Central Dogma of Molecular Biology. Chem, 7(11), 2883–2895. https://doi.org/10.1016/j.chempr.2021.09.014
- Wong, F., & Gunawardena, J. (2020). Gene Regulation in and out of Equilibrium. Annual review of biophysics, 49, 199–226. https://doi.org/10.1146/annurev-biophys-121219-081542
- Goh, H. H., Ng, C. L., & Loke, K. K. (2018). Functional Genomics. Advances in experimental medicine and biology, 1102, 11–30. https://doi.org/10.1007/978-3-319-98758-3_2