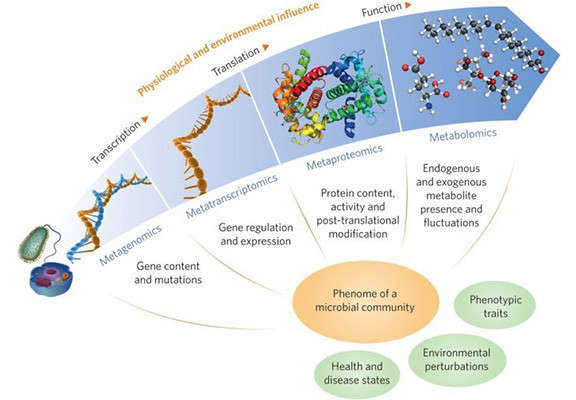
Gene expression—the process that controls how and when genes are turned on or off—is a key concept in biology and medicine. It helps explain how living things grow, develop different cell types, adapt to their surroundings, and stay balanced. This article looks at the many steps involved in controlling gene expression, from how tightly DNA is packed in the cell to how RNA is processed and proteins are made and modified. It covers both simple organisms like bacteria (prokaryotes) and more complex ones like humans (eukaryotes), pointing out what makes their systems similar or different. It also explores new technologies that are helping scientists better understand genes and develop treatments in fields like medicine and biotechnology.
The Background and Significance of Gene Expression
Since the mapping of the human genome, scientists have recognized that only a portion of the estimated 20,000 to 25,000 protein-coding genes are actively expressed in a given cell type. This selective utilization is orchestrated by a network of finely tuned regulatory mechanisms that ensure cellular specificity and functional diversity despite a common genomic template.
Gene expression regulation encompasses the controlled synthesis of RNA and proteins in terms of timing, spatial localization, and abundance. Regulatory mechanisms operate at numerous biological levels, including:
- Structural modifications to chromatin
- Transcription initiation and elongation
- RNA processing and transport
- mRNA translation
- Protein modification and degradation
Together, these layers maintain cellular homeostasis, support organismal development, and mediate responses to environmental cues. Dysregulation at any level can contribute to disease pathology, underlining the importance of these mechanisms in both health and therapeutic intervention.
Multilayered Control of Gene Expression Regulation
Chromatin-Level Expression Regulation
1. Chromatin Structure and Remodeling Complexes
DNA is packaged into chromatin, a complex of DNA and histone proteins. The accessibility of DNA for transcription depends on the chromatin state—whether it is in a compacted (heterochromatin) or relaxed (euchromatin) form. Chromatin remodelers, such as SWI/SNF and ISWI complexes, utilize ATP to reposition or evict nucleosomes, thereby exposing regulatory DNA elements. These remodelers work in concert with transcription factors to modulate gene activation or repression.
2. Epigenetic Marks and Histone Variants
Epigenetic modifications, including DNA methylation and histone tail modifications (e.g., acetylation and methylation), act as signals that regulate gene accessibility. Histone variants such as H2A.Z and H3.3 can also influence chromatin dynamics. For instance, trimethylation of histone H3 at lysine 4 (H3K4me3) is associated with active promoters, whereas H3K27me3 correlates with gene silencing. Long non-coding RNAs like XIST recruit epigenetic complexes such as PRC2 to specific genomic loci, leading to targeted silencing. These processes are integral to development, memory formation, and cancer progression.
Transcriptional Expression Regulation
1. DNA Regulatory Elements (Cis-Elements)
Gene transcription is directed by a repertoire of DNA regulatory elements, including:
- Core promoters with TATA and Inr motifs for basal transcription factor binding.
- Proximal promoter elements (e.g., CAAT box, GC-rich regions).
- Enhancers, which activate transcription at distant promoters through chromatin looping.
- Silencers and insulators, such as CTCF-binding sites, which modulate boundary activity and repressive chromatin domains.
These sequences provide specificity to transcription factor binding and support combinatorial control mechanisms.
2. Protein-Based Regulators (Trans-Acting Factors)
Transcription factors fall into two primary classes:
- General transcription factors (e.g., TFIIA, TFIIB, TFIID) that assemble the pre-initiation complex with RNA polymerase II
- Sequence-specific regulators (e.g., Myc, Sp1, estrogen receptor) that bind DNA motifs and modulate transcriptional output
Mediator complexes bridge transcription factors and polymerases, enabling context-sensitive gene expression. Mutations in regulatory proteins such as TP53 and RUNX1 are frequently implicated in carcinogenesis and developmental anomalies.
3. Initiation and Elongation Processes
Transcriptional initiation begins with the assembly of a pre-initiation complex and often features a regulatory checkpoint known as promoter-proximal pausing. This pause, controlled by NELF and DSIF, can be released by P-TEFb, enabling productive elongation.
Termination involves polyadenylation signals and cleavage factors that process the nascent transcript. Alternative polyadenylation contributes to transcriptomic complexity, particularly in immune and nervous systems.
 Figur 1. Key steps of gene transcription.( Cramer P, 2019)
Figur 1. Key steps of gene transcription.( Cramer P, 2019)
Post-Transcriptional Expression Regulation
1. Alternative Splicing Mechanisms
Alternative splicing diversifies the proteome by allowing multiple transcript isoforms to be derived from a single gene. Regulatory proteins such as SR factors and hnRNPs influence splice site selection, modulated by both sequence elements and RNA secondary structures. Misregulated splicing is associated with malignancies and inherited disorders.
2. Post-Transcriptional RNA Editing
Editing of RNA sequences post-transcription enhances molecular diversity:
- ADAR-mediated adenosine-to-inosine conversion alters codon identity, particularly in neurons.
- APOBEC enzymes mediate cytidine-to-uridine transitions in transcripts like APOB, modifying lipid metabolism.
These modifications are tightly regulated and evolutionarily conserved.
3. Stability and Degradation of mRNA
mRNA longevity affects protein synthesis rates. Key determinants include:
- 5' cap and 3' poly-A tail integrity.
- AU-rich elements in 3' UTRs that interact with RNA-binding proteins (RBPs).
- microRNA (miRNA)-guided RISC complexes that trigger degradation or translational repression.
Stress granules and processing bodies serve as transient mRNA repositories or degradation sites.
 Figur 2. Modes of RNA binding.( Hentze, M. W. et al, 2018)
Figur 2. Modes of RNA binding.( Hentze, M. W. et al, 2018)
4. Intracellular mRNA Distribution
Certain transcripts are transported to specific subcellular locales through interaction with RBPs that recognize "zipcode" sequences. For example, localized translation at neuronal synapses is critical for plasticity and memory formation. Live imaging approaches, such as MS2 tagging, allow real-time tracking of mRNA dynamics.
Translational Expression Regulation
1. Mechanisms of Translation Initiation
Translation typically begins via cap-dependent scanning mechanisms involving the eIF4F complex. Regulatory influences include:
- mTOR signaling, which modulates availability of eIF4E via 4E-BP binding.
- Internal ribosome entry sites (IRES) for cap-independent initiation under stress.
- Upstream open reading frames (uORFs) that affect main coding sequence translation.
These controls ensure adaptability during nutrient deprivation or cellular stress.
2. Elongation and Termination Nuances
Elongation rates are influenced by codon usage bias, tRNA pools, and mRNA structure. Ribosome stalling serves functional roles in co-translational folding and protein targeting. Stop codon readthrough and frameshifting contribute to proteomic expansion.
3. Non-Coding RNA Influence on Translation
Non-coding RNAs refine translational control:
- miRNAs inhibit initiation or elongation through 3' UTR interactions.
- lncRNAs and circRNAs serve as miRNA sponges or modulate translation factors.
These interactions form intricate post-transcriptional networks impacting gene output.
 Figur 3. Levels of regulation of microRNA expression.( Gulyaeva, L. F. et al, 2016)
Figur 3. Levels of regulation of microRNA expression.( Gulyaeva, L. F. et al, 2016)
Post-Translational Expression Regulation
1. Reversible Protein Modifications
Post-translational modifications (PTMs) alter protein properties:
- Phosphorylation cascades (e.g., MAPK pathways) adjust activity.
- Acetylation and methylation affect chromatin association and transcriptional regulation.
- Ubiquitination targets proteins for proteasomal degradation.
- Glycosylation and SUMOylation influence trafficking and stability.
PTM profiles are mapped using high-resolution mass spectrometry.
2. Protein Folding and Degradation Pathways
Proteostasis is maintained through:
- Molecular chaperones like HSPs that assist folding.
- ER-associated degradation (ERAD) to eliminate misfolded proteins.
- Autophagy for degrading aggregated or long-lived proteins.
Failures in these systems are implicated in neurodegeneration and proteinopathies.
3. Cellular Trafficking and Localization
Proteins are targeted to cellular compartments via signal sequences:
- N-terminal signal peptides guide proteins to the ER or mitochondria.
- Nuclear localization signals (NLS) and nuclear export signals (NES) govern nucleocytoplasmic exchange.
- Lipid anchors (e.g., prenylation) associate proteins with membranes.
Correct trafficking is essential for function, particularly in polarized cells.
Expression Regulation in Prokaryotic Regulatory Systems
- (1)Operons and Transcriptional Logic
In bacteria, functionally linked genes are often arranged in operons. Regulation involves:
- Repressor proteins (e.g., LacI) and inducers (e.g., allolactose) in lac operon.
- Feedback repression and attenuation in trp operon.
Such arrangements allow efficient, coordinated control of related pathways.
- (2)Sigma Factors and Transcriptional Specificity
RNA polymerase holoenzyme specificity is conferred by sigma factors:
- RpoD for housekeeping genes
- RpoH for heat shock response
- RpoS for stationary phase genes
Sigma factor competition determines transcriptional priorities under changing conditions.
- (3)Post-Transcriptional Control in Bacteria
Regulatory elements include:
- Shine-Dalgarno sequences for ribosome binding.
- Riboswitches that sense metabolites to influence translation or transcription termination.
- sRNAs that modulate target mRNA stability or translation.
These mechanisms support adaptive responses and synthetic biology applications.
Expression Regulation in Biological and Clinical Relevance
- (1)Role in Embryonic Development
Patterning of the embryo relies on spatial gene expression gradients (e.g., Bicoid in Drosophila). Key regulators such as HOX, Pax6, and Nanog orchestrate tissue specification and organogenesis.
- (2)Determination of Cellular Identity
Differentiation involves tissue-specific transcription factors (e.g., GATA1 in erythropoiesis) and chromatin remodeling. Single-cell RNA sequencing has revealed cell-type heterogeneity and dynamic transitions.
- (3)Environmental Adaptation and Homeostasis
Cells adjust gene expression in response to:
- Heat shock via HSF1 activation
- Hypoxia via HIF1α signaling
- Nutrient changes via SREBP and ChREBP
- Circadian cues via CLOCK/BMAL1 networks
These responses sustain physiological balance and resilience.
- (4)Association with Human Diseases
Gene misregulation is implicated in numerous pathologies:
- Cancers: epigenetic silencing of tumor suppressors, activation of oncogenes.
- Developmental disorders: mutations in regulatory genes (e.g., MECP2 in Rett syndrome).
- Metabolic syndromes: disruption of nuclear receptor pathways (e.g., PPARγ, LXR).
Expression signatures inform diagnostics, prognosis, and therapeutic targeting.
Expression Regulation Technologies and Applications
(1)Emerging Technologies
- RNA-seq and long-read Iso-Seq: transcriptomic profiling.
- ChIP-seq and ATAC-seq: chromatin and accessibility mapping.
- Hi-C and 3C: 3D genome architecture.
- Ribo-seq: ribosome occupancy mapping.
- CRISPR interference (CRISPRi): transcriptional repression.
- Single-cell omics: resolution of rare populations and developmental trajectories.
(2)Biomedical Applications
- Gene therapy using siRNAs, antisense oligonucleotides, and viral vectors.
- Epigenetic drugs targeting DNA methyltransferases and histone deacetylases.
- Stratified medicine based on gene expression classifiers.
- Custom regulatory networks in engineered cells for biosensing and therapeutic delivery.
Future Perspectives on Expression Regulation
Future research will emphasize:
- Integration of multi-omics datasets for comprehensive regulatory mapping.
- Functional annotation of non-coding genomic elements.
- Time-resolved studies of regulatory dynamics during development and disease.
- Single-molecule and spatial transcriptomics for in situ analysis.
- Ethical frameworks and safeguards for genome editing technologies.
A systems-level perspective on gene regulation will enable predictive models of cellular behavior and inform the next generation of therapeutic innovations.
Conclusion
The regulation of gene expression encompasses a rich, multilayered system vital to all aspects of biology. Its complexity is balanced by precision, enabling diverse phenotypes from a common genome. Continued exploration, powered by technological progress and interdisciplinary collaboration, will further unravel the intricacies of gene regulation and unlock novel opportunities in science and medicine.
References
- Cramer P. (2019). Organization and regulation of gene transcription. Nature, 573(7772), 45–54. https://doi.org/10.1038/s41586-019-1517-4
- Hentze, M. W., Castello, A., Schwarzl, T., & Preiss, T. (2018). A brave new world of RNA-binding proteins. Nature reviews. Molecular cell biology, 19(5), 327–341. https://doi.org/10.1038/nrm.2017.130
- Gulyaeva, L. F., & Kushlinskiy, N. E. (2016). Regulatory mechanisms of microRNA expression. Journal of translational medicine, 14(1), 143. https://doi.org/10.1186/s12967-016-0893-x