Overview of HLA
Human leukocyte antigen (HLA) molecules are essential components of the immune system, playing a crucial role in activating antitumor immune responses through their interactions with T cell receptors (TCRs). HLA molecules are a class of glycoproteins composed of an alpha-heavy chain and a beta-light chain that are non-covalently bound. The structure of HLA molecules positions the amino terminus of the peptide chain outward, while the carboxyl terminus extends into the cytoplasm, with the hydrophobic region residing within the cytosol. The distribution and function of HLA molecules lead to their classification into two main types: HLA class I antigens and HLA class II antigens.
The Human Leukocyte Antigen (HLA) gene complex is indeed located on the short arm of chromosome 6 at the position 6p21.31. This complex is a crucial part of the immune system and plays a central role in immune response regulation.
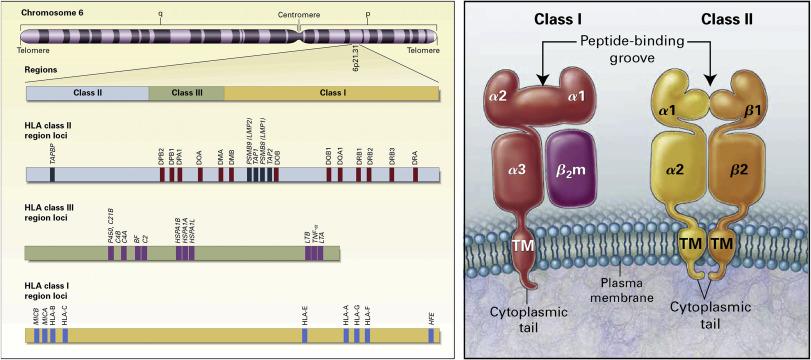
Genetic organization of the human leukocyte antigen (HLA) complex. (Frenet et al., 2019)
- HLA Class I Antigens & Genes
HLA class I antigens are present on the surface of virtually all nucleated cells in the body. These antigens play a critical role in immune recognition and response. When a foreign substance, such as a virus or a chemical agent, alters the gene products of the host cells, these modified HLA class I antigens can become targets for immune recognition and subsequent elimination. The altered HLA class I antigens act as autoimmunogens, triggering immune responses against the modified cells. The frequently examined sites for HLA class I genes encompass the alpha chains that are encoded by the A, B, and C loci.
- HLA Class II Antigens & Genes
In contrast, HLA class II antigens are primarily expressed on the surface of specialized immune cells known as antigen-presenting cells (APCs), including B cells, macrophages, and activated T cells. The primary role of HLA class II molecules is to facilitate the recognition and presentation of exogenous antigens to helper T cells (Th cells). When these antigens are presented, they bind to the co-receptor CD4 on the surface of Th cells, which in turn helps regulate and direct immune responses. The engagement of HLA class II antigens in antigen presentation is crucial for initiating immune responses against invading pathogens. Genetic testing for HLA class II primarily targets the alpha and beta chains coded by the DR, DQ, and DP loci.
- HLA Class III genes
This group includes genes that encode proteins involved in immune response regulation and inflammation, such as components of the complement system and cytokines.
- HLA Expression and Antitumor Immunity
HLA molecules and their expression patterns are of utmost importance in the context of antitumor immunity. HLA class I antigens enable the immune system to identify and target cells that have undergone transformations due to viral infection or other pathological changes. This recognition allows cytotoxic T cells to recognize and eliminate these aberrant cells, thus contributing to antitumor immune surveillance.
HLA Gene Polymorphisms
(i) Diverse Motifs: Arising from variations in HLA gene compositions and resultant expression products, a multitude of functionally distinct motifs emerge.
(ii) Varied Alleles: The HLA gene clusters manifest heterogeneity and polymorphic variations across populations, encompassing disparate alleles.
(iii) Co-Dominant Expression: The phenomenon of co-dominant expression, wherein all alleles are individually expressed, augments the richness of HLA polymorphism within populations.
(iv) Genetic Transcription and Regulation: Allelic-dependent discrepancies in cis-acting elements exert influence over gene transcription processes, contributing to allele-specific regulation.
High-Resolution HLA Typing through Sequencing
- HLA Typing by Next-Generation Sequencing (NGS)
The achievement of robust and accurate HLA typing utilizing Next-Generation Sequencing (NGS) methodologies is contingent upon the judicious enhancement of specific target human leukocyte antigen (HLA) loci. This augmentation is effectuated through either the application of polymerase chain reaction (PCR)-based amplification or the utilization of bait-based capture strategies. Conventionally, the resultant enriched DNA targets undergo a process of stochastic fragmentation prior to sequencing, followed by subsequent comprehensive analytical assessment. Techniques such as bait-based capturing, long-range PCR, or the generation of shorter PCR amplicons spanning the region of interest are selectively employed to meticulously interrogate the enriched targets.
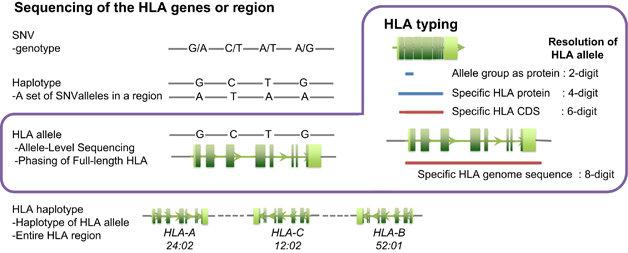
HLA typing to provide sequencing data for the HLA gene(s) and regions. (Hosomichi et al., 2015)
PCR, while precise in its targeted enrichment, occasionally yields off-target sequences during NGS, necessitating extensive target coverage. Nonetheless, PCR remains susceptible to allelic dropouts, often triggered by single nucleotide polymorphisms (SNPs) at primer sites, suboptimal primer sets, or amplification biases. In contrast, the bait-based approach ameliorates allelic dropout limitations, as even slight mismatches between bait and target DNA enable effective hybridization.
- HLA Typing by Long-Read Sequencing
Conversely, NGS alone may prove insufficient for elucidating comprehensive HLA gene haplotypes. To surmount these constraints, we introduce an end-to-end workflow leveraging long-read sequencing, PscBio Single Molecule Real-Time (SMRT) technology, and Nanopore sequencing for HLA class I and II gene sequencing. This methodology empowers high-resolution HLA typing and allele-specific HLA expression determination.
Applications of HLA Typing
(1) Significance in Organ Transplantation
Within clinical spheres, the foremost impediment to triumphant allogeneic transplantations (barring cases involving homozygous twins) is the formidable challenge of graft rejection. The inheritance of Major Histocompatibility Complex (MHC) adheres to Mendelian principles, delineating three distinctive scenarios concerning HLA similarity—namely, complete concordance, partial likeness, and outright divergence—amongst siblings. Renal transplants from sibling donors boasting congruent HLA profiles exhibit a success rate surpassing 90%, while outcomes for those with partial congruence markedly diminish. Transplants involving disparate HLA profiles yield notably diminished survival rates for recipients.
(2) Forensic Utility
The HLA system, acknowledged for its enduring genetic constancy, emerges as an exemplar genetic marker epitomizing individual distinctiveness. This distinctiveness is preserved throughout one’s lifetime owing to the elevated polymorphism present in the HLA genes. The likelihood of HLA type concordance between unrelated individuals remains exceedingly low, thereby rendering it an indispensable tool in matters of paternity determination.
(3) Implications in Oncology
The influence of HLA-mediated maladies extends decisively into the realm of oncogenesis, directly contributing to the promotion of cancer progression.
(4) Diagnosis and Therapeutic Prospects
Genetic polymorphisms constitute the underpinning genetic substrate governing interindividual disparities in drug responsiveness. Through the identification of associations between specific polymorphic genotypes and drug efficacy as well as toxicity profiles, the groundwork is laid for the emergence of personalized therapeutic strategies.
References
- Frenet, Emeline Masson, and Andromachi Scaradavou. "Human Leukocyte Antigens." Transfusion Medicine and Hemostasis. Elsevier, 2019. 191-197.
- Hosomichi, Kazuyoshi, et al. "The impact of next-generation sequencing technologies on HLA research." Journal of human genetics 60.11 (2015): 665-673.


 Sample Submission Guidelines
Sample Submission Guidelines