An Overview of Single-Cell Genomics:Introduction, Key Technologies and Challenges
Single-cell genomics employs high-throughput sequencing technologies to delve into the genome, transcriptome, epigenome, and proteome of individual cells, uncovering cellular heterogeneity and paving new avenues for disease research and precision medicine. Compared to traditional multi-cell analyses, this technology effectively circumvents information loss, offering an unprecedented perspective on understanding cellular functions and complex biological systems. Despite existing challenges in cell capture, sequencing depth, and data integration, continuous technological breakthroughs are expected to enhance the pivotal role of single-cell genomics in precision medicine and disease treatment, propelling life science research to new heights.
Introduction of Single-Cell Genomics
Single-Cell Genomics represents a cutting-edge approach that leverages high-throughput sequencing to meticulously examine the genome, transcriptome, epigenome, and proteome at the individual cell level.
What is single cell genomics
The field of individual cell genomic analysis focuses on examining genetic material at the cellular level. Within this discipline, researchers investigate temporal variations in genetic expression patterns, genomic alterations, modifications of the epigenome, and fluctuations in cellular protein content. By analyzing discrete cells rather than aggregate samples, this methodological approach circumvents the averaging effects that typically obscure detailed insights in conventional bulk analyses, thereby offering unprecedented granularity in our understanding of both cellular mechanisms and broader biological processes.
A fundamental contribution of individual cellular genomics stems from its capacity to elucidate variation among cells within identical tissues or cellular populations. Such biological diversity manifests across multiple dimensions, encompassing distinctive patterns of gene activity, regulatory mechanisms in the epigenome, metabolic processes, and specialized cellular roles. This capability has proven particularly valuable in oncological investigations, where technologies for sequencing individual cells enable detailed characterization of diverse cellular components within tumors, facilitating the identification of specific subpopulations that promote cancer progression.
Application areas of single-cell genomics
Single-cell genomics has a wide range of applications, covering many fields such as cancer research, neuroscience, and immunology. The following are some specific application examples:
(1) Cancer research
Single cell sequencing technology is revolutionary in cancer research. It can resolve the heterogeneity within the tumor, including tumor stem cells, cancer cell subsets, and immune cells in the tumor microenvironment. This information helps identify potential treatment targets and guides the development of precision medicine strategies.
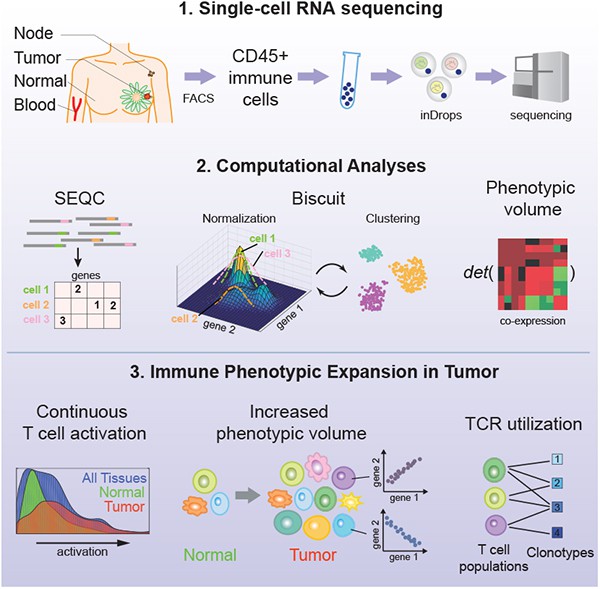 Figure 1.Single-cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment.(Azizi, E.,et.al,2018)
Figure 1.Single-cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment.(Azizi, E.,et.al,2018)
(2) Neuroscience
In the field of neuroscience, single-cell genomics can resolve the functional heterogeneity of neurons and glial cells, thereby helping to understand the mechanisms of nervous system development, disease occurrence, and neural signal transmission.
Resource
- Single-cell RNA Sequencing: Introduction, Methods, and Applications
- Single-cell RNA Sequencing: Quality Control
- Single-Cell Transcriptome Research Methods and Applications
- Transcriptomic Research: Single-Cell and Spatial Transcriptomics
- A Guide of Single-Cell DNA Methylation Sequencing: From Wet Experiment to Data Analysis
- High-throughput Single-cell V(D)J Sequencing: Introduction, Principle and workflow
(3) Immunology
The application of single cell technology in immunology can analyze the diversity of immune cells and their role in disease. For example, analyzing the gene expression patterns of individual immune cells can reveal the complexity of the immune response and provide a theoretical basis for the design of immunotherapy.
(4) Developmental biology
In developmental biology, single-cell genomics can interpret the molecular mechanisms in cell fate determination and differentiation pathways, helping to reveal the complex processes of tissue formation and organ development.
(5) Human genetics
Single-cell genomics also has important application value in human genetics. For example, by analyzing genomic information from individual cells, the impact of genetic variations on an individual's phenotype can be revealed and new ideas for the diagnosis and treatment of rare genetic diseases can be provided.
Key Technologies in Single-Cell Genomics
Single-cell genomics has revolutionized our understanding of cellular diversity by enabling the analysis of individual cells. Key technologies in this field include:
Single-Cell RNA Sequencing (scRNA-Seq)
scRNA-Seq technique profiles the transcriptomes of individual cells, allowing researchers to identify gene expression patterns and discover rare cell types within a population. It involves isolating single cells, extracting and amplifying RNA, preparing sequencing libraries, and sequencing using next-generation platforms.
Single-Cell DNA Sequencing
Single-cell DNA sequencing (scDNA-seq) is used to analyze genomic information from individual cells, including mutations, copy number variations (CNVs), and chromosomal structural variations (SCNV). Compared to traditional population sequencing, scDNA-seq provides high-resolution data on the genetic background of individual cells. This technique is particularly suitable for studying tumor heterogeneity, genetic diseases, and genomic changes during cell development.
Single-Cell Epigenome Sequencing
Single cell ATAC sequencing (scATAC-seq) is a technique used to analyze the accessibility of chromatin in individual cells and reveals the open state of gene regulatory regions. By combining transposase-mediated reversible end hybridization (REPT) technology, scATAC-seq can capture open areas of chromatin and generate a chromatin accessibility map. scATAC-seq plays an important role in studying gene regulatory networks, epigenetic changes, and cell state transitions. For example, it can help identify transcription factors and regulatory pathways associated with specific diseases.
Spatial Transcriptomics
Spatial Transcriptomics is a cutting-edge technology that combines single-cell transcriptomics and spatial information to simultaneously analyze gene expression and their spatial distribution in tissues. This technology reveals the location of cells in tissues and their functional relationships by integrating single-cell resolution gene expression data with spatial coordinates in tissue slices, providing a new perspective for understanding cell heterogeneity, tissue structure and disease mechanisms.
Techniques for Single-Cell Isolation
Single cell isolation technology is an important tool in modern biological and medical research, used to extract individual cells from complex cell populations for analysis. The following are detailed descriptions of several common single cell isolation techniques:
Microfluidic Technologies
Microfluidic technology is a technology that controls liquid flow based on tiny channels and is widely used in single cell separation. The main methods include:
Droplet-based Systems: Use a water-in-oil (O/W) or oil-in-water (W/O) biphasic system to encapsulate individual cells in microliter droplets. This method has the advantages of high throughput, low cross-contamination and easy subsequent analysis.
Micro-chips and arrays: Capture and processing of single cells through microchannels or microarrays on microchips. These devices often incorporate automated capabilities to efficiently isolate and analyze single cells.
The advantages of microfluidic technology are its high throughput, portability and automation, but it relies on externally driven pumps and valves, and the equipment cost is high.
Laser Capture Microdissection(LCM)
Laser capture microdissection is a single cell isolation method based on laser technology. The laser accurately cuts a target area on a tissue section to obtain individual cells or cell clusters.
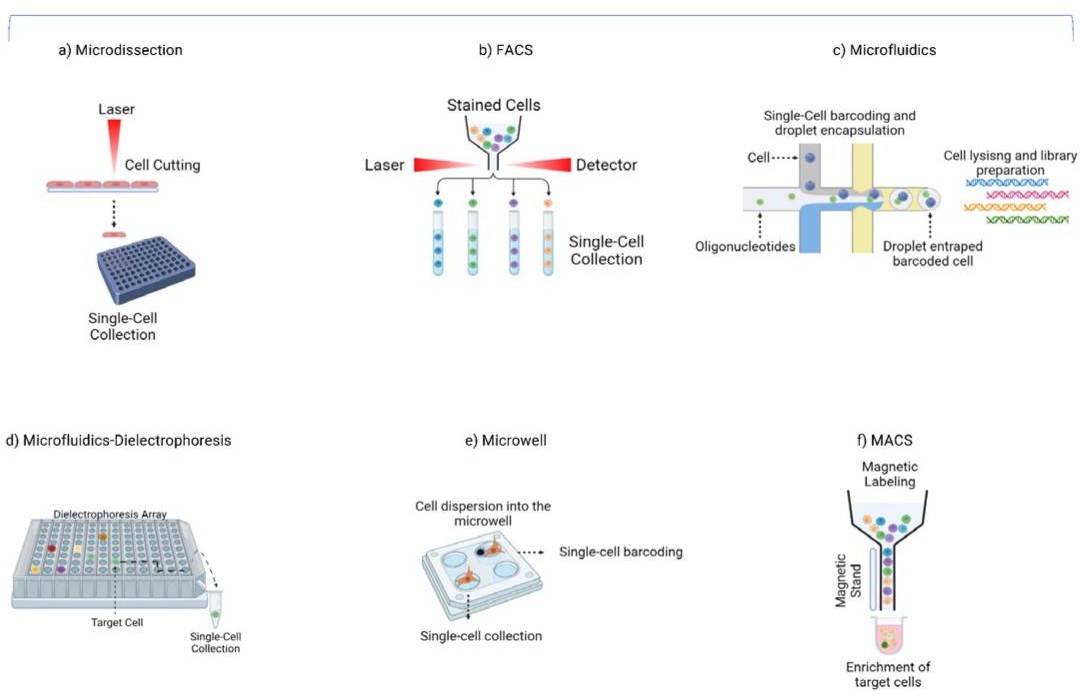 Figure 2.Single-cell LCM isolation methods.(Massimino, M.,et.al,2023)
Figure 2.Single-cell LCM isolation methods.(Massimino, M.,et.al,2023)
The advantage of LCM is its ability to maintain the integrity and morphology of the cells, but its operation is complex and costly, and often requires highly skilled operators.
Fluorescence-Activated Cell Sorting( FACS)
FACS is a flow cytometry technology that achieves high-throughput separation of single cells through fluorescent labeling and laser detection. FACS is capable of simultaneously analyzing multiple parameters and selectively isolating target cells based on specific markers. This method is suitable for scenarios requiring high purity and efficient separation, but the equipment is expensive and the operation is complex.
| Technology Name | Advantages | Disadvantages |
|---|---|---|
| Microfluidic Technology | High throughput, automation, low cross-contamination | Requires external driving equipment, high cost |
| Laser Capture Microdissection (LCM) | High precision, preserves cell integrity | Complex operation, high cost |
| Fluorescence-Activated Cell Sorting (FACS) | High throughput, high purity, multi-parameter analysis | Expensive equipment, complex operation |
These technologies are widely used in fields such as genomics, transcriptomics, cancer research, neuroscience, and personalized medicine. For example, in liver cancer diagnosis, LCM and FACS are used to extract single cancer cells from tissue samples for analysis; while in single cell sequencing, microfluidic technology and FACS are commonly used single cell isolation methods.
The choice of single cell isolation technology depends on research needs, sample types, and budget constraints. Microfluidic technology has become the mainstream direction of future development due to its high-throughput and automation characteristics, while FACS and LCM still have irreplaceable advantages in specific fields.
Challenges in Single-Cell Genomics
Single-cell genomics is of great value in studying cell heterogeneity and complex biological systems, but its development also faces many challenges.
Experimental technical challenges
Cell capture and sample preparation: Single cell sequencing requires the isolation and amplification of genetic material from a single cell. However, loss of genetic material is prone to occur during the cell capture process (such as incomplete DNA extraction), and the single cell sample size is very small (only two copies of DNA), resulting in amplification bias and Allelic loss. Although the development of new technologies such as microfluidic has improved capture efficiency, further optimization is still needed.
Sequencing depth and data quality: Insufficient sequencing depth for single cell sequencing can lead to technical "dropout"(low-expressed genes are not detected)
, and affects data saturation (that is, it is difficult to increase after the amount of sequencing read information stabilizes). Although increasing the depth of sequencing can alleviate the problem, it is costly. In addition, the quality of sequencing reagents and equipment performance will also affect data accuracy.
Pollution and noise :Technical noise may be introduced due to RNA/DNA cross-contamination or operating errors during the experiment, and the biological heterogeneity of a single cell itself (such as gene expression fluctuations) may also increase data complexity.
Data integration and computing challenges
Single-cell genomics involves multimodal data (such as transcriptome, genome, epigenome, and proteomic data), and the integration and analysis of these data is an important challenge in current research:
Computational complexity of high-dimensional data: Single cell data usually appears as a high-dimensional feature space, which poses huge computational challenges to data analysis. For example, how to preserve the relative relationships between cells in low-dimensional space is a key issue.
Data integration issues: Data integration between different experimental conditions, sample types and measurement methods requires the development of new algorithms and tools. For example, how to batch correct single cell data from different experiments or correlate transcriptome accessibility with genetic variation.
Sparsity and missing data processing: A large number of observations of zero (sparsity) are common in scRNA-seq data, which makes data analysis more difficult. In addition, due to technical reasons or biological characteristics, a large number of missing values often appear in single cell data
Solutions and future directions
To address the above challenges, the researchers have proposed various solutions:
Improve capture and amplification efficiency: Improve the efficiency and accuracy of single cell capture by improving microfluidic technology and automated equipment.
Reduce the risk of contamination: Optimize sample processing processes, such as using non-polluting lysis reagents and strict laboratory operating specifications.
Improve sequencing depth and quality: Increase sequencing saturation and reduce noise by optimizing sequencing strategies and using high-quality reagents.
Develop efficient data integration algorithms: Leverage advanced bioinformatics tools such as batch correction methods and low-dimensional embedding techniques to integrate multimodal data.
Address the computing challenges of high-dimensional data: Introduce new dimensionality reduction techniques (such as t-SNE, UMAP) and machine learning algorithms to process high-dimensional data and extract meaningful information.
Data Analysis and Bioinformatics in Single-Cell Genomics
Single-cell genomics data analysis and bioinformatics is a rapidly developing field. Its core goal is to analyze cell heterogeneity, gene expression patterns, and cell function through high-resolution single-cell level data. However, research in this area faces many challenges, especially in terms of data integration, development of analytical tools, and comprehensive analysis of multi-omics data.
Overview of computing tools and platforms
Data analysis for single-cell genomics relies on multiple computing tools and platforms that are capable of processing data from single-cell sequencing (such as single-cell RNA sequencing, single-cell ATAC sequencing, etc.). For example, Seurat Label Transfer and LIGER are two commonly used computing tools used to integrate single-cell transcriptome, epigenomic and spatial transcriptome data to reveal complex disease mechanisms. In addition, with the development of single-cell multi-omics technology, researchers can simultaneously capture the genome, transcriptome, epigenome and proteomic information of a single cell, which provides a new perspective for a deeper understanding of cell states.
In recent years, machine learning methods (such as probability graphical models) have been widely used in the analysis of single cell data to improve data modeling and visualization capabilities. In addition, deep learning technology has also shown advantages in the integration of single-cell multi-omics data, although there is currently a lack of systematic research.
Challenges of interpreting large and complex data sets
Single cell data is characterized by high dimensionality and noise, which makes data analysis particularly complex. For example, single-cell RNA sequencing data often has low genomic coverage and high amplification bias, which makes data analysis more difficult. In addition, the sparsity of single cell data and the heterogeneity between cells also put forward higher requirements for data processing.
In actual operation, data pretreatment is one of the key steps, including standardization, gene selection, batch correction and cluster analysis.
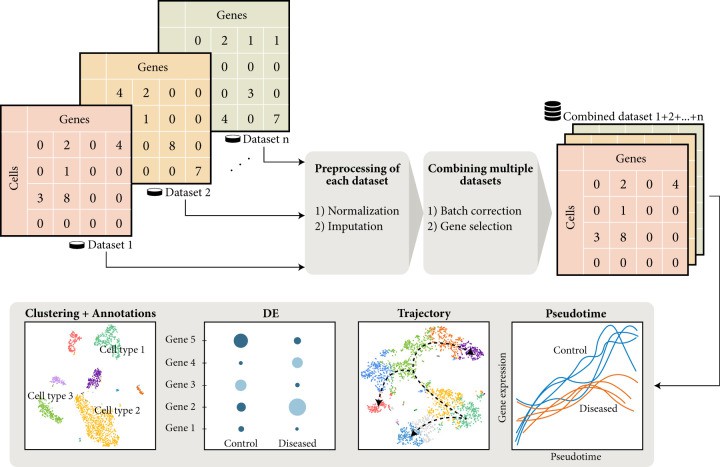 Figure 3 .Overview of single-cell data analysis workflow.(Auerbach, B. J.,et.al,2021)
Figure 3 .Overview of single-cell data analysis workflow.(Auerbach, B. J.,et.al,2021)
However, due to the complexity of single-cell data, many traditional bioinformatics tools are not suitable for this type of data. Therefore, it is particularly important to develop calculation methods specifically for single cell data.
Integrated analysis with multi-omics data
Recent advances in technologies enabling multi-omics analysis at the single-cell level have substantially expanded research possibilities while simultaneously creating new challenges in data consolidation. A key scientific question involves the optimal methods for combining and analyzing diverse molecular datasets, including genomic, transcriptomic, and epigenomic information, to comprehensively understand cellular states. Scientists have developed various approaches to address these integration challenges, with solutions ranging from proximity-based algorithms that utilize weighted distance measurements (notably anchor-based techniques) to sophisticated frameworks built on deep learning principles.
However, these methods still face some limitations. For example, heterogeneity between different modal data and batch size effects may affect the accuracy of integrated results. In addition, how to preserve spatial information during the integration process is also an important research direction.
Conclusion
As a revolutionary technology, single cell genomics is profoundly transforming biomedical research and clinical practice. It has shown great potential in revealing cell heterogeneity, promoting the development of precision medicine, and promoting research on disease mechanisms. In the future, with the continuous advancement of technology and the deepening of multidisciplinary cross-cooperation, single cell genomics will play a more important role in the fields of precision medicine, disease treatment and biotechnology.
References:
- Azizi, E., Carr, A. J., Plitas, G., Cornish, A. E.,et.al. (2018). Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell, 174(5), 1293–1308.e36. https://doi.org/10.1016/j.cell.2018.05.060
- Massimino, M., Martorana, F., Stella, S., et.al. (2023). Single-Cell Analysis in the Omics Era: Technologies and Applications in Cancer. Genes, 14(7), 1330. https://doi.org/10.3390/genes14071330
- Auerbach, B. J., Hu, J., Reilly, M. P., & Li, M. (2021). Applications of single-cell genomics and computational strategies to study common disease and population-level variation. Genome research, 31(10), 1728–1741. https://doi.org/10.1101/gr.275430.121


 Sample Submission Guidelines
Sample Submission Guidelines
