In the vast theatre of nature, microorganisms reign supreme as the oldest and most abundant life forms, holding a pivotal position. A question often arises: "Why do microorganisms in similar environments evolve differently?" The answer lies in the intricate and fascinating process of divergent evolution. Despite seemingly identical environmental conditions, microorganisms can evolve into populations with distinct morphologies, physiological traits, and ecological functions—a phenomenon that has piqued the interest of many biologists. Delving into how microorganisms adapt to their niches through divergent evolution, and the roles played by genetic and environmental factors in this process, holds incalculable value for our understanding of life’s diversity and ecosystem stability.
This article aims to dissect the mechanisms of microbial divergent evolution and ecological adaptation. It comprehensively explores how microorganisms, in similar environments, accumulate genetic differences among populations through a complex interplay of genetic and environmental factors, ultimately adapting to diverse niches.
Divergent Evolution in Microorganisms
Divergent evolution in microorganisms, in simple terms, refers to the gradual accumulation of genetic differences among microbial populations in similar environments. Within the same ecological niche, different microbial groups are not static; instead, throughout prolonged evolution, due to the interplay of various internal and external factors, their genomes change, leading to genetic disparities among populations. These differences are not coincidental but rather crucial means by which microorganisms adapt to their environment and gain a survival advantage. Through divergent evolution, microorganisms can better utilize limited resources, avoid excessive competition with other microbes in the same environment, and thus occupy unique ecological niches within the ecosystem. There are several driving factors behind microbial divergent evolution.
Horizontal Gene Transfer
Horizontal gene transfer plays a pivotal role in microbial divergent evolution. It provides microorganisms with a rapid means of acquiring new traits, with antibiotic resistance being the most quintessential example. In environments where antibiotics are widely used, some microorganisms acquire resistance genes from others through horizontal gene transfer, enabling them to survive under antibiotic stress. This rapid dissemination and integration of genes leads to significant genetic changes within microbial populations within a short period, accelerating the process of divergent evolution. For instance, in hospital settings, the emergence of multidrug-resistant bacteria is often closely linked to horizontal gene transfer. Different strains exchange resistance genes through mobile genetic elements such as plasmids and transposons, resulting in resistant strains gradually dominating the population.
Accumulation of Mutations
In isolated microbial populations, the accumulation of neutral mutations serves as a significant driving force behind divergent evolution. Due to geographical isolation or other factors, microbial populations become fragmented into relatively isolated groups. Within these groups, genetic mutations occur randomly, and since the effects of natural selection are relatively weak, some neutral mutations gradually accumulate within the population. Over time, these accumulated mutations lead to an increasing genetic divergence between different isolated populations, thereby propelling the process of divergent evolution.
Environmental Pressures
Environmental pressures are potent drivers of microbial divergent evolution. Factors such as nutrient availability, temperature, pH, and competition all influence microbial survival and evolution. When nutrient resources in the environment are limited, microorganisms evolve distinct metabolic pathways to adapt to this resource-scarce environment. For example, some microorganisms in oligotrophic environments have evolved efficient nutrient uptake and utilization mechanisms, enabling them to grow using substrates that other microorganisms cannot utilize.
Changes in temperature and pH also prompt microorganisms to evolve corresponding adaptive traits. In high-temperature environments, microorganisms may evolve heat-resistant enzymes and proteins to maintain normal physiological functions; in acidic or alkaline environments, they regulate intracellular pH to adapt to external environmental changes. Additionally, competition among microorganisms also drives divergent evolution. To gain a competitive edge, microorganisms continually evolve novel survival strategies, such as producing antibiotics to inhibit the growth of other microorganisms or evolving specialized cell surface structures to evade predation by other microbes.
Case Studies on Divergent Evolution
In the field of microbiology research, the phenomenon where microorganisms exhibit vastly different characteristics despite inhabiting similar environments has long captured attention. Despite sharing seemingly identical ecological backgrounds, diverse microbial species can evolve remarkably distinct morphologies, physiological functions, and ecological habits. Divergent evolution plays a pivotal role in this unique phenomenon. To gain a deeper understanding of how microorganisms adapt to different ecological niches through divergent evolution, as well as the roles played by genetic and environmental factors in this process, we will now delve into specific case studies to further illuminate the significance of divergent evolution in microbial ecological adaptation.
Wang et al. conducted a study on the functional differentiation and horizontal gene transfer (HGT) of denitrifying bacteria within anammox (anaerobic ammonium oxidation) bacterial communities through the lens of microbial divergent evolution. The researchers extracted 77 high-quality metagenome-assembled genomes (MAGs) of denitrifying bacteria from sludge samples obtained from a pig farm wastewater treatment plant. They discovered significant functional differentiation among these bacteria in terms of carbohydrate utilization and vitamin synthesis. For instance, certain denitrifying bacteria possessed fewer genes for biotin, pantothenic acid, and methionine synthesis compared to their counterparts in other habitats.
Furthermore, the study revealed that genes related to energy production and inorganic ion transport might have been horizontally transferred into denitrifying bacteria, while these bacteria could also donate nutrient transporter genes to other microorganisms, facilitating their utilization of new metabolites. The research underscores the crucial role of horizontal gene transfer and the presence of mobile genetic elements in the adaptive evolution of denitrifying bacteria. It elucidates the mechanisms underlying functional differentiation within anammox bacterial communities, offering fresh perspectives on the evolution and functionality of microbial communities.
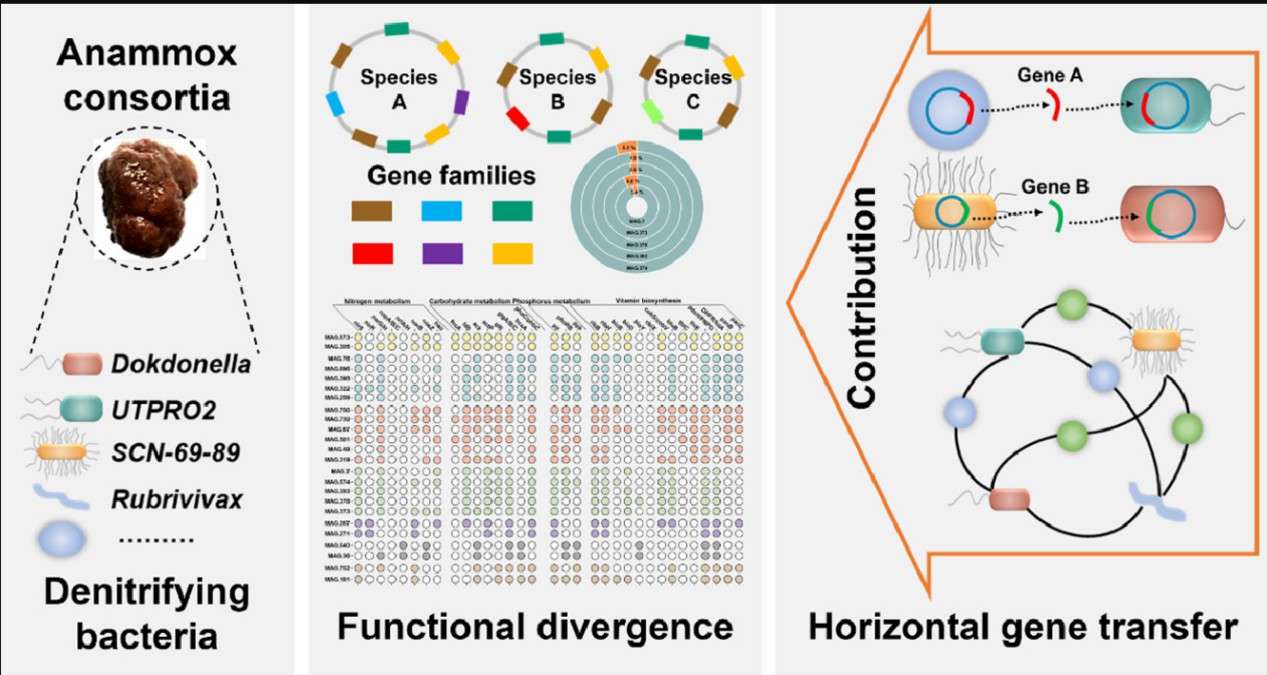
Convergent evolution of denitrifying bacteria (Wang et al., 2022)
Wang and colleagues delved into the adaptive evolution of uncultured bacteria within anaerobic ammonium oxidation (anammox) communities by leveraging microbial divergent evolution. Utilizing PacBio HiFi long-read sequencing technology, they obtained 30 complete metagenome-assembled genomes (cMAGs) from anammox granular sludge samples taken from a laboratory-scale bioreactor. These cMAGs represented nine distinct phyla, including one novel anammox species belonging to Candidatus Jettenia and three species from newly identified families.
The study revealed functional differentiation among anammox community members in both general and nitrogen metabolism, suggesting potential collaboration through cross-feeding strategies in the metabolism of organic compounds, cofactors, and vitamins. Additionally, the researchers identified 63 mobile genetic elements (MGEs) and 50 potential horizontal gene transfer (HGT) events, indicating the significant role of HGT and MGEs, particularly transposons containing tnpA, in the adaptive evolution of anammox bacteria. This research not only provides a theoretical foundation for understanding the ecological roles and biotechnological applications of anammox bacterial communities but also showcases the advantages of HiFi sequencing technology in studying complex microbial communities.

Promoter sequence in the prokaryotic cell (Prakash et al., 2023)
Ma and colleagues investigated the adaptive responses of the anaerobic ammonium-oxidizing (anammox) bacterium Kuenenia stuttgartiensis to zinc ion (Zn(II)) stress through metatranscriptomic analysis. The research was prompted by the frequent presence of Zn(II) in high-concentration wastewater treatment systems, yet the cytotoxic mechanisms of Zn(II) on anammox bacteria remained unclear. Utilizing a highly enriched culture of Kuenenia stuttgartiensis, the researchers analyzed its responses to Zn(II) concentrations of 50, 100, and 150 mg/L. Their findings revealed that while anammox activity decreased with increasing Zn(II) concentrations, the number of viable cells remained stable. Through metatranscriptomic analysis, they discovered that Kuenenia stuttgartiensis employs a complex regulatory network to cope with Zn(II) stress, encompassing functions such as substrate degradation, Zn(II) efflux, chelation, DNA repair, protein degradation, synthesis, and signal transduction.
Notably, the bacterium upregulated genes encoding the RND efflux family (e.g., czcA, czcB, czcC) for active Zn(II) efflux. These genes could serve as "sentinel genes" to detect the initial inhibitory phase of Zn(II) on anammox bacteria. This study provides a theoretical basis for developing early warning indicators to predict the risk of operational failure in anammox bioreactors due to Zn(II) shock.

Convergent evolution of the anaerobic ammonia-oxidising bacterium Kuenenia stuttgartiensis under zinc ion (Zn(II)) stress(Ma et al., 2020)
Molecular Mechanisms Underlying Divergent Evolution
The remarkable diversity of ecological adaptations observed in microbial divergent evolution is underpinned by a series of intricate and complex molecular mechanisms. These mechanisms regulate genetic variation and expression at the gene level, enabling microorganisms to embark on distinct evolutionary trajectories in similar environments and adapt to their unique ecological niches. Below, we delve into the key molecular mechanisms governing microbial divergent evolution, unveiling the mysteries of how microorganisms achieve this at the genetic level.
Genomic Islands
Genomic islands are regions of horizontally transferred genetic material that encode niche-specific functions, playing a pivotal role in microbial divergent evolution. These islands often carry genes associated with specific environmental adaptations, such as antibiotic resistance genes, heavy metal resistance genes, and metabolic genes. When microorganisms encounter specific environments, they can acquire these genomic islands through horizontal gene transfer, thereby gaining the ability to thrive in those conditions.
For instance, in soils contaminated with heavy metals, certain microorganisms can survive in high-concentration metal environments by acquiring genomic islands that carry heavy metal resistance genes. The transfer and accumulation of such genomic islands lead to pronounced genetic differences among microbial populations across various ecological niches, driving the occurrence of divergent evolution.
CRISPR-Cas Systems
CRISPR-Cas systems serve as adaptive immune mechanisms in microorganisms, enabling them to fend off phage invasions by acquiring spacer sequences. When microbial populations face phage pressure, variations in how their CRISPR-Cas systems acquire these spacers drive adaptation to the phage threat. For instance, in environments teeming with phages, microbial CRISPR-Cas systems become more active, continually acquiring new spacers to bolster their resistance. This differential spacer acquisition leads to genomic divergence among microbial populations, thereby promoting divergent evolution. Moreover, CRISPR-Cas systems may also play roles in other microbial physiological processes, such as gene expression regulation, further influencing microbial ecological adaptability.
Epigenetic Regulation
Epigenetic regulation also plays a pivotal role in microbial divergent evolution. Under stressful conditions, microbial strains like Escherichia coli exhibit variations in DNA methylation. These modifications can impact gene expression patterns, allowing microorganisms to swiftly adapt to environmental changes. For example, when E. coli encounters nutrient-depleted environments, the methylation status of certain genes changes, leading to their upregulation or downregulation. This enables the microorganism to adjust its metabolic pathways and enhance its survival capabilities. Epigenetic regulation provides microorganisms with a rapid response mechanism to environmental shifts, working in tandem with genetic variations to drive the process of divergent evolution.
Advanced Tools for Studying Divergent Evolution
Microbial divergent evolution involves intricate interactions between genetics and the environment, with underlying patterns and mechanisms often hidden within the interplay of microscopic genetic changes and macroscopic ecological phenomena. To precisely unravel these mysteries, traditional research methods alone are insufficient. With the rapid advancement of science and technology, a suite of advanced research tools has emerged, acting as precise "scalpels" that enable us to delve deeper and more meticulously into every detail of microbial divergent evolution. Let’s embark on a journey into the world of these advanced tools and explore how they assist us in unveiling the secrets of microbial divergent evolution.
Long-read sequencing technologies, such as PacBio and Nanopore sequencing, offer unique advantages in deciphering structural variations within microbial biosynthetic gene clusters (BGCs) responsible for secondary metabolite production. BGCs are critical genomic regions where microorganisms synthesize secondary metabolites, and structural variations within these clusters are often closely linked to microbial ecological adaptability. Long-read sequencing provides longer read lengths, enabling accurate identification of insertions, deletions, inversions, and other structural variations within BGCs.
This crucial genomic information is instrumental in studying microbial divergent evolution. For instance, when investigating the divergent evolution of antibiotic-producing strains, long-read sequencing can help identify differences in BGCs among various strains, thereby revealing the evolutionary mechanisms underlying their antibiotic synthesis capabilities.
Single-Cell Metagenomics
Single-cell metagenomics technologies, such as the MobiMicrobe platform, offer powerful tools for analyzing rare microbial strains. In complex environmental samples, many microbial strains are challenging to study using traditional methods due to their low abundance. Single-cell metagenomics enables genome sequencing of individual microbial cells, thereby unveiling the genetic characteristics and ecological functions of rare strains. For instance, in soil microbial community studies, single-cell metagenomics has allowed us to discover rare strains with unique metabolic capabilities, providing new leads for the development and utilization of microbial resources.
Ancestral State Reconstruction
Ancestral state reconstruction techniques, such as Bayesian inference, can be employed to study the evolution of ecological traits in Prochlorococcus. Prochlorococcus is a significant group of marine microorganisms, with different ecotypes exhibiting variations in morphology, physiology, and ecological functions. By utilizing ancestral state reconstruction, we can trace the evolutionary history of these traits across Prochlorococcus ecotypes, revealing the mechanisms underlying their divergent evolution. For example, research has shown that Prochlorococcus, in response to varying marine environmental pressures, gradually forms ecotypes adapted to different ecological niches through genetic mutations and selection.

Convergent evolution of microorganisms
Conclusion
The study of microbial divergent evolution and ecological adaptation mechanisms holds profound and far-reaching significance. From a fundamental scientific perspective, it offers a unique lens through which to understand the diversity of life and the evolutionary journey, unveiling how microorganisms have adapted to changing environments over eons through unique strategies. In practical applications, the findings of this research provide fresh perspectives and methodologies for addressing numerous real-world challenges. For instance, in environmental pollution control, we can leverage the principles of microbial divergent evolution to screen and cultivate microbial strains with specific degradation capabilities, enabling more efficient treatment of various pollutants. In the realm of disease prevention and control, a deep understanding of microbial divergent evolution mechanisms aids in predicting the mutation trends of pathogenic microorganisms, enabling the proactive formulation of targeted prevention and control strategies.
Looking ahead, with ongoing advancements in research and continuous technological innovation, we anticipate unraveling even more mysteries of microbial divergent evolution. This will pave the way for making greater contributions to the sustainable development and well-being of human society.
References
- Wang D, Meng Y, Meng F. "Genome-centric metagenomics insights into functional divergence and horizontal gene transfer of denitrifying bacteria in anammox consortia." Water Res. 2022; 224:119062. https://doi.org/10.1016/j.watres.2022.119062
- Wang YC, Mao Y, Fu HM, Wang J, Weng X, Liu ZH, Xu XW, Yan P, Fang F, Guo JS, Shen Y, Chen YP. "New insights into functional divergence and adaptive evolution of uncultured bacteria in anammox community by complete genome-centric analysis." Sci Total Environ. 2024; 924:171530. https://doi.org/10.1016/j.scitotenv.2024.171530
- Ma X, Yan Y, Wang W, Guo J, Wang Y. "Metatranscriptomic analysis of adaptive response of anammox bacteria Candidatus ‘Kuenenia stuttgartiensis’ to Zn(II) exposure." Chemosphere. 2020; 246:125682. https://doi.org/10.1016/j.chemosphere.2019.125682


 Sample Submission Guidelines
Sample Submission Guidelines