Functional Genomics Analysis of Plant Pathogens: From Mechanistic Insights to Disease Management and Control
By integrating PacBio/Nanopore, multiomics and CRISPR gene editing technology, Functional genomics of plant pathogens systematically analyzes the pathogenic mechanism and host interaction network of fungi, oomycetes and other pathogens. It has been found that pathogens target and interfere with plant immune pathways through adaptive evolutionary strategies such as the spatio-temporal specific expression of effector proteins and horizontal gene transfer. Host-induced gene silencing (HIGS) and targeted editing of disease-causing genes provide new means for precise intervention. To address the challenge of genome complexity, spatial transcriptomics and machine learning have significantly improved the integration accuracy of multi-omics data, revealing the dynamic changes of secondary metabolic gene clusters and other key pathogenic features. At the application level, breeding for disease resistance based on the effector protein-NLR interaction mechanism and the development of small molecule inhibitors targeting disease-causing genes are promoting the innovation of high-resistance crop varieties and green pesticides. In the future, interdisciplinary integration and pathogen evolution prediction models will optimize the disease monitoring network, providing theoretical and technical support for building an intelligent prevention and control system and ensuring food security.
Introduction to plant pathogens and functional genomics
Classification and hazards of plant pathogens
Plant pathogens mainly include fungi, oomycetes, bacteria, viruses and nematodes. Their infection mechanisms are diverse, posing a serious threat to global agriculture. Fungal pathogens, such as Magnaporthe oryzae, destroy host cells by secreting effector proteins and toxins, causing rice blast and resulting in 10%-30% crop loss. Oomyces use RxLR effector proteins to suppress plant immunity, causing the Irish famine in the 19th century. Bacterial pathogens rely ona type III secretion system to deliver virulence factors that induce systemic diseases such as citrus canker. Plant viruses, such as tobacco Mosaic virus (TMV), are transmitted by vector insects and interfere with host gene expression, resulting in leaf deformities and decreased yield. Nematodes invade roots through oral needles to form giant cells that impede water and nutrient uptake. Pathogens cause more than $220 billion in crop losses globally each year, according to the FAO, and climate change increases the risk of disease spread. Typical examples include citrus greening (a bacterial disease), which has devastated citrus industries in several countries, and a new strain of wheat stem rust, Ug99, which threatens global food security. Elucidation of the functional genomic characteristics of these pathogens is a key basis for developing targeted control strategies.
Service you may interested in
Definition of functional genomics
Functional genomics is an important discipline in the post-genomic era, aiming to systematically analyze the biological functions of genes and their products, their regulatory networks, and their associations with phenotypes, going beyond mere sequence analysis to reveal "how genomes work." Different from structural genomics (which focuses on gene localization and sequencing), functional genomics integrates multi-dimensional data such as transcriptome, proteome, epigenome and metabolome, combined with gene editing (e.g. CRISPR-Cas9), gene silencing (RNAi) and other technologies. Dynamic study of gene expression patterns, interactions, and regulatory mechanisms under specific physiological or pathological conditions. In the study of plant pathogens, functional genomics provides theoretical basis for targeted prevention of disease by identifying disease-related genes (such as effector proteins, toxin synthesis genes) and resolving host-pathogen interaction molecular networks (such as immunosuppressive mechanisms). For example, the pathogenic function of effector proteins can be verified by gene knockout, or the key regulatory hubs in infection can be revealed by the spatiotemporal transcriptome. The field is driving innovation in disease resistance breeding and precise prevention and control technology, and is a bridge connecting genomic information with the actual function of organisms.
The core issues of genomic analysis of plant pathogens
Plant pathogen genomics focuses on five core issues: 1) identification of pathogenic genes: screening of effector proteins, toxin synthesis genes and other key factors, and analyzing the molecular mechanisms of their interference with host immunity or induction of cell death; 2) host-pathogen interaction mechanism: elucidate the recognition rules of pathogenic model molecules (PAMPs) and plant PRR receptors, as well as the "gene-to-gene" specific interaction between effector proteins and host NLR disease resistance proteins; 3) Adaptive evolutionary drivers: reveal how horizontal gene transfer, expansion of effector protein families, and genome rearrangement enhance pathogen virulence (e.g., rapid evolution of RxLR effector protein in Oomyces ovulus); 4) Multi-omics dynamic regulation: integrating spatiotemporal transcriptome, epigenome and metabolome to analyze the gene expression network and metabolic interaction during infection; 5) Technology transformation bottleneck: Overcome complex genome (polyploid, high repeat sequence) assembly problems, using CRISPR editing and machine learning (such as AlphaFold) to develop targeted prevention and control strategies (disease resistance breeding, small molecule inhibitors). The above research aims to systematically analyze the pathogenic mechanism, and provide theoretical and technical support for the precise prevention and control of agricultural diseases.
Functional genomics research methods and techniques
Genome sequencing and annotation
Genome sequencing and annotation are the cornerstones of functional genomics research. Based on the advantages of three-generation sequencing technology with high precision and long read length, the complex genome of pathogens can be deciphered, and gene function annotation can be completed in combination with homology alignment and structure prediction. Comparative genomics can reveal the expansion of disease-related gene families, horizontal gene transfer events, and host adaptive evolution through cross-species or intraspecific strain comparisons. Transcriptomics (RNA-seq, single-cell sequencing) analyze the dynamic expression of genes at different stages of infection and locate key pathogenic regulatory hubs. Epigenomics focuses on epigenetic marks such as DNA methylation and histone modification to elucidate mechanisms of silencing or activation of virulent genes in pathogens. Proteomics uses mass spectrometry to identify the secretion pathways of effector proteins and their interaction networks with host target proteins. Metabolomics tracks metabolite exchange in pathogen-host interactions and reveals mechanisms of synthesis and regulation of toxins. Multi-omics data integration to build a gene-protein-metabolite multi-dimensional regulatory model, providing a systematic perspective for pathogenesis analysis and target mining.
Technology of gene function verification
Gene function verification techniques confirm the biological role of target genes by targeting manipulation and phenotypic associations. CRISPR-Cas9 gene editing accurately knocks out or taps into pathogen genes to directly verify their pathogenic functions; RNA interference (RNAi) and HIGS can specifically silence pathogen genes through double-stranded RNA, which has both mechanism research and disease-resistance application value. Heterologous expression systems introduce target genes into model organisms, and verify functional independence by inducing phenotypes; Phenotypic complementation experiments further confirm gene function by restoring the mutant phenotype by complementing genes. Fluorescent labeling and in vivo imaging trace the temporal and spatial dynamics of gene products in real time, revealing molecular interactions during infection. The integration of multiple omics data assisted in the screening of candidate genes, and analyzed the regulatory network of genes in host-pathogen interactions in combination with the above technical systems, providing experimental basis for the discovery of disease resistance targets and precise intervention, and promoting the research of pathogen pathogenesis and the innovation of prevention and control strategies.
Functional genomic characteristics of plant pathogens
Classical Effectors and pathogenetic mechanisms
Effector proteins are key toxic factors secreted by pathogens and promote infection by interfering with host cell function. Fungal and oomycetes effectants enter host cells via specific domains that target suppression of immune signaling pathways: for example, blocking plant PTI (PAMP-triggered immunity) associated kinase activity, or inducing programmed cell death in the host. Bacterial effector proteins are injected into plant cells via a type III secretion system to modify host proteins to inhibit defense responses. Viral effector proteins hijack host translation mechanisms to ensure viral genome replication. The pathogenetic mechanisms of effector proteins are highly synergistic: early effector proteins inhibit basic immunity, while late effector proteins manipulate host metabolism. For example, the effector protein PsXEG1 of Phytophthora impairs cell wall defense by inhibiting host glycosylhydrolase activity; Meanwhile, its homologous suppressor PsXLP1 can protect PsXEG1 from host enzymatic hydrolysis, forming an "attack and defense" strategy. Studying the interaction between effector proteins and host NLR disease resistance proteins revealed the "gene-to-gene" disease resistance model and provided a target for disease resistance breeding. Rapid evolution of effector proteins is the core strategy of pathogens to evade host recognition. Elucidation of their functions and regulatory networks lays a foundation for developing effectorprotein targeting inhibitors or disease resistance gene editing.
Classic case studies
Tomatoes are one of the most important vegetable crops in the world and one of the most well-studied cultivated dicotyledonous plants. It is often used as a model species for plant studies, including classical genetics, cytogenetics, molecular genetics, and molecular biology.
Recently, Horticulture Research has published online a review article by Maria Doroteia Campos et al., of the University of Evora in Portugal, systematically summarizing important studies using the high-throughput RNA-seq technique to obtain tomatoes in response to a wide range of pathogen changes. Such as transcriptome studies in response to viruses, fungi, bacteria, oomycetes, and nematodes. Understanding the network of plant genes involved in activating disease-resistant gen responses is critical to developing molecular tools for disease resistance. In order to fully understand the regulatory pathways induced by pathogen infection in plants, potential differentially expressed genes are shown in Figure 1 (Fig. 1).
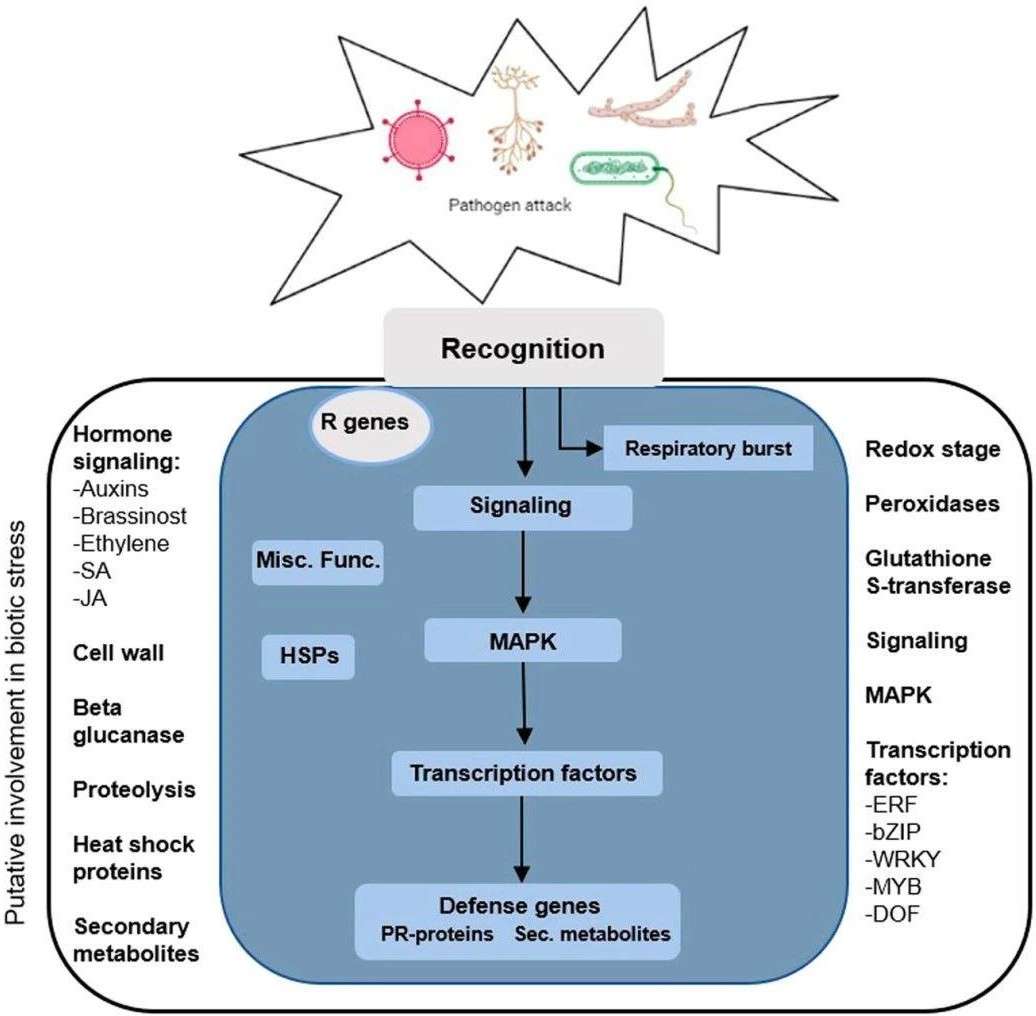 Figur1 . High throughput sequencing unravels tomato-pathogen interactions towards a sustainable plant breeding (Maria DC.2021)
Figur1 . High throughput sequencing unravels tomato-pathogen interactions towards a sustainable plant breeding (Maria DC.2021)
Recently, College of Agriculture and Biology and College of Life Science and Technology of Shanghai Jiao Tong University jointly published new research results in Nature Communications. They isolated Pseudomonas P. mosselii 923 from rice rhizosphere soil, revealing the biosynthesis and regulation mechanism of plant pathogen antagonistic active substance pyrazoltriazine. The strain specifically inhibited the growth of Xanthomonas oryzae and blast fungus, providing a broader choice for the control of rice pathogens.
Technical challenges and solutions driven by functional genomics
Functional genomics research challenges for plant pathogens
Functional genomics of plant pathogens faces multiple challenges: 1) Genome complexity: pathogen genomes often contain high repeat sequence, polyploid structure (such as rust bacteria) or dynamic chromosomal variation, resulting in difficulties in three-generation sequencing assembly and annotation; 2) The dynamics of host-pathogen interactions: the infection process involves spatio-temporal specific gene expression and metabolic interactions, requiring the combination of single cell and spatial transcriptome to improve resolution; 3) Multi-omics data integration bottleneck: massive genome, epigenome and metabolic data require the development of novel algorithms to build regulatory networks and reveal the key hubs of pathogenesis; 4) Limitations of functional verification technology: genetic transformation efficiency of some pathogens is low (such as oomycetes), CRISPR editing and in vivo imaging technology still need to be optimized; 5) Application transformation barriers: there is a gap between pathogenic mechanism analysis and field prevention and control, such as effector protein targeting inhibitors can easily lead to pathogen resistance evolution. In addition, the rapid adaptive evolution of pathogens (such as the expansion of effector protein gene families) requires research that is both timely and predictive. Overcoming these challenges requires interdisciplinary integration (AI prediction, synthetic biology) and technological innovation to facilitate the implementation of precise prevention and control strategies.
Precursor of other biomolecules
Lysine is not only a building block for proteins but also a precursor for many bioactive molecules. First, lysine is a precursor for carnitine synthesis. Carnitine is essential for fatty acid metabolism, transporting long-chain fatty acids into mitochondria for β-oxidation and energy production. Lysine is converted into carnitine through a series of enzymatic reactions requiring cofactors like vitamin C and iron. Carnitine deficiency disrupts fatty acid metabolism, impairing energy production. Second, lysine is a precursor for certain bioactive molecules. For example, lysine can be decarboxylated to form cadaverine, a polyamine involved in cell growth and differentiation. Cadaverine can be further metabolized into other polyamines, such as spermidine and spermine, which play roles in DNA stability, gene expression, and cell proliferation. Additionally, lysine is involved in nicotinic acid (vitamin B3) synthesis. Nicotinic acid is a precursor for NAD+ and NADP+, coenzymes critical for redox reactions, energy metabolism, and cell signaling. Lysine is converted into nicotinic acid via the kynurenine pathway, highlighting its significance in metabolism.
Breakthroughs in cutting-edge Technologies
In recent years, many technological breakthroughs have been made in functional genomics research of plant pathogens: 1) endogenous CRISPR system development: based on the pathogen's own CRISPR-CAS system (such as bacterial bacterial type I-C), combined with λ-Red recombination technology to achieve efficient gene editing and large fragment deletion, greatly simplifying the operating process8; 2) viral vector delivery technology: the use of engineered plant viruses (such as positive strand RNA viruses, twin virus replicons) to deliver gene editing elements, breaking through the bottleneck of traditional genetic transformation, and significantly improving the efficiency of precision editing 1; 3) Multi-omics integration and microbiome analysis: Building the world's first crop rhizosphere microbial genome database (CRBC/CRVC), combining metagenomic and metabolome data to reveal the "functional symbiosis alliance" of rhizosphere microorganisms and the potential of phage directed regulation 6; 4) Regulation mechanism of non-coding RNA: For the first time in filamentous fungi, long-chain non-coding RNA (e.g. Fusarium oryzae RNA5P) regulated toxin synthesis gene (TRI5), revealing the "double insurance" regulatory network5 of secondary metabolism5; 5) Machine learning and AI prediction: Based on AlphaFold prediction of the three-dimensional structure of the effect protein, combined with metagenomic data analysis, to accelerate disease gene mining and disease target design. These technological breakthroughs provide multi-dimensional tool support for the analysis of pathogenic mechanism of pathogens and the development of green prevention and control strategies.
Application and translational research
The results of functional genomics research on plant pathogens are rapidly being translated into agricultural prevention and control practices. In the field of disease resistance breeding, based on the interaction mechanism between effector protein and host NLR disease resistance protein, the use of gene editing (CRISPR) to create broad-spectrum disease-resistant crops; In the development of targeted fungicides, small molecule inhibitors (such as compounds targeting the secretion of RxLR effector proteins) are designed by analyzing toxin synthesis pathways or effector protein functional sites; In terms of disease monitoring, the establishment of pathogen genome Database (such as Phytophthora Database), combined with CRISPR-Cas12a rapid molecular detection technology, to achieve real-time tracking of pathogen virulence variation in the field; These transformation applications significantly reduce the dependence on chemical pesticides, improve crop disease resistance resilience, and in the future, combined with synthetic biology and AI prediction models, will accelerate the construction of intelligent prevention and control systems.
Conclusion
Functional genomics of plant pathogens provides a revolutionary perspective for the prevention and control of agricultural diseases by systematically analyzing the functions of pathogenic genes and their interaction networks with hosts. Its core significance lies in: 1) Revealing the molecular mechanism of disease: elucidating how key factors such as effector proteins and toxin synthesis genes inhibit plant immunity or hijack metabolic pathways, providing theoretical basis for targeted intervention; 2) Drive disease resistance technology innovation: based on the "gene-to-gene" interaction model, the use of gene editing (such as CRISPR modification of NLR receptor) to create broad spectrum disease-resistant varieties, reduce the dependence on chemical pesticides; 3) Promote green prevention and control strategies: achieve precise disease prevention and control through small-molecule inhibitors targeting disease-causing genes, RNA biopesticides (such as HIGS technology) and microbiome regulation.
Reference:
- Campos, M.D., Félix, M.d.R., Patanita, M. et al. (2021). High throughput sequencing unravels tomato-pathogen interactions towards a sustainable plant breeding. Hortic Res, 8, 171. https://doi.org/10.1111/j.1399-3011.1972.tb03424.x


 Sample Submission Guidelines
Sample Submission Guidelines
