Overview of Immune Defense Against Pathogens
The immune system operates like a precisely timed orchestra, with innate and adaptive immunity as its dual conductors. Innate immunity acts as the first responder, launching immediate but generalized attacks. Macrophages patrol tissues, using Toll-like receptors (TLRs) to scan for molecular patterns unique to invaders-like bacterial lipopolysaccharides (LPS) or viral RNA. When TLR4 detects a Gram-negative bacterium, it triggers NF-κ signaling within minutes, releasing cytokines to summon neutrophils. This rapid response resembles casting a wide net, indiscriminately trapping threats but lacking precision.
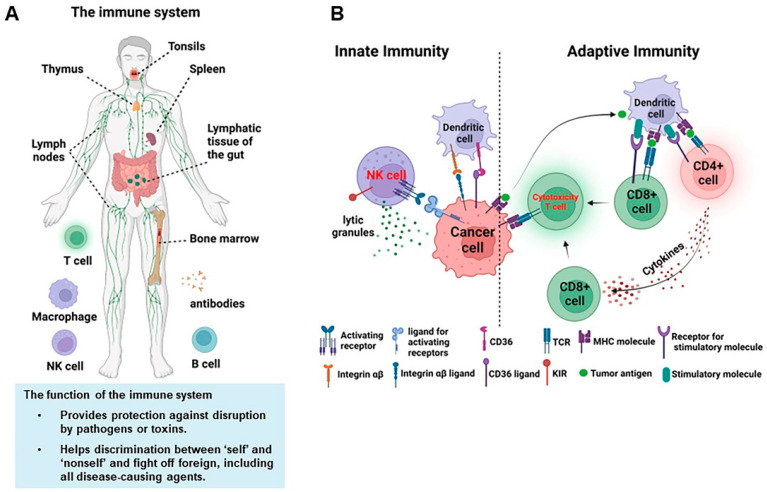 The human immune system and its functions. (Yu et al., 2023)
The human immune system and its functions. (Yu et al., 2023)
Adaptive immunity, in contrast, operates like a specialized strike force. T and B cells generate billions of receptor variants through genetic shuffling, creating a vast library of potential pathogen blueprints. Dendritic cells act as intelligence officers, presenting pathogen fragments to T cells. Successful recognition sparks targeted cloning into killer T cells or antibody-producing B cells. Modern vaccines, such as those targeting SARS-CoV-2, leverage this system by training B cells to produce antibodies that block viral entry.
These systems collaborate intricately. Innate immune signals guide adaptive responses: TLR4 activation releases IL-12, directing T cells toward Th1 specialization for intracellular threats like tuberculosis. Conversely, TLR2 activation prompts Th17 cells to fortify mucosal barriers against fungi. γδ T cells exemplify this synergy, bridging innate and adaptive roles by recognizing both generic and specific pathogen markers. However, imbalances-like excessive innate responses overwhelming adaptive defenses in sepsis-highlight the fragility of this partnership.
Pathogens Detection Mechanisms
Toll-like receptors (TLRs) and cytoplasmic sensors play crucial roles in detecting pathogens, triggering innate immune responses and shaping adaptive immunity through distinct pathways and mechanisms.
The role of toll-like receptors
TLRs function as molecular sentinels stationed at cellular gateways. Surface-bound TLR4 detects bacterial LPS, while endosomal TLR3 intercepts viral RNA. Binding triggers distinct pathways: TLR4 recruits MyD88 to activate NF-κB and inflammatory genes, whereas TLR3 uses TRIF to initiate antiviral interferon production. Zika virus studies reveal critical role of TLR3-mice lacking this receptor suffer severe neurological damage.
Cytoplasmic sensors add redundancy. NOD-like receptors (NLRs) detect bacterial peptidoglycan, assembling inflammasomes to activate IL-1β and induce fever. Meanwhile, RIG-I receptors identify viral RNA signatures, like the 5' triphosphate group in influenza genomes, triggering mitochondrial MAVS proteins to amplify interferon alerts. This layered surveillance ensures no pathogen goes unnoticed-even if one sensor fails, others compensate.
TLRs also shape adaptive strategies. TLR4-activated dendritic cells secrete IL-12 to guide T cell differentiation, while TLR2/1 recognition of mycobacterial lipopeptides drives Th17 development. Such "signal translation" enables innate immunity to instruct adaptive responses, as seen in parasitic infections where TLR11 directs antibody class-switching to IgE.
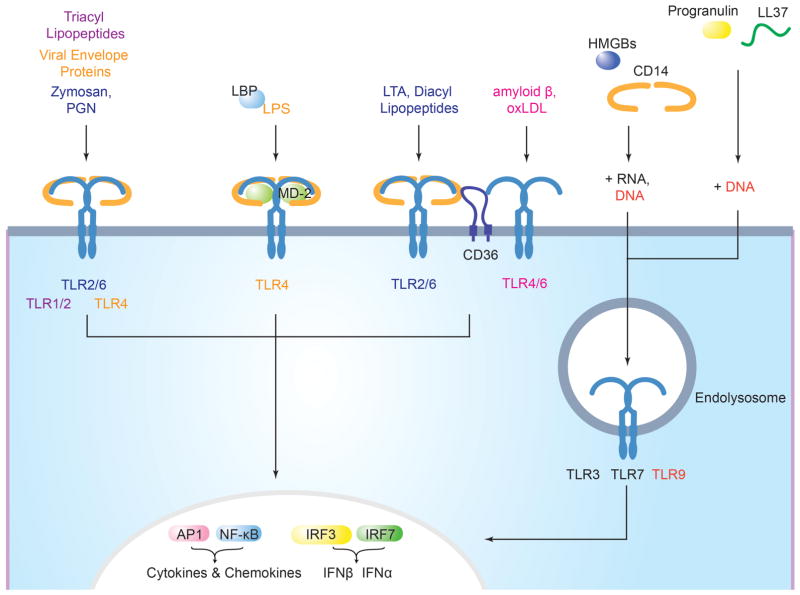 Accessory molecules mediate ligand binding and delivery to surface and endosomal TLRs. (Lee et al., 2012)
Accessory molecules mediate ligand binding and delivery to surface and endosomal TLRs. (Lee et al., 2012)
The function of macrophages and neutrophils
Macrophages serve as tissue guardians, combining phagocytosis with antigen presentation. Upon encountering Staphylococcus aureus, TLR2 recognition triggers phagosome formation and reactive oxygen species (ROS) to dismantle bacteria. Crucially, macrophages display processed antigens on MHC-II molecules, "teaching" T cells to recognize threats-a process vital for controlling tuberculosis, where IFN-γ from Th1 cells activates macrophage autophagy.
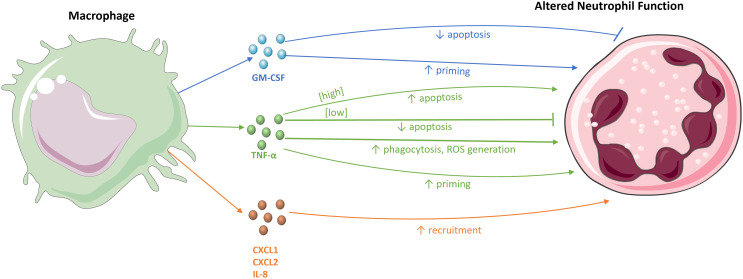 The effects of macrophages on neutrophils. (Gaffney et al., 2022)
The effects of macrophages on neutrophils. (Gaffney et al., 2022)
Neutrophils execute rapid strikes. Within hours of a Streptococcus pneumoniae lung invasion, IL-8 guides them through blood vessels to engulf pathogens. They deploy hypochlorous acid (akin to household bleach) in phagosomes and, when overwhelmed, release neutrophil extracellular traps (NETs)-DNA webs laced with antimicrobial peptides. In necrotizing fasciitis, NETs entangle bacteria at the cost of the neutrophil's life.
Their collaboration is double-edged. Macrophage-derived TNF-α primes neutrophil migration, while neutrophil elastase amplifies macrophage inflammation. However, dysregulation-like in severe COVID-19-sparks cytokine storms where overactive neutrophils damage lung tissue, illustrating the peril of unchecked teamwork.
Real-life Examples of Detecting Pathogens
During a Streptococcus pyogenes skin infection, macrophages detect bacterial lipoteichoic acid via TLR2, releasing IL-1β to recruit neutrophils. These cells squeeze through blood vessels, deploying ROS and NETs to contain the threat. Meanwhile, influenza viruses face dual scrutiny: alveolar macrophages detect viral RNA via RIG-I, prompting interferon-driven "antiviral lockdown," while cytotoxic T cells target infected cells displaying viral HA fragments.
Pathogen diversity demands tailored responses. Mycobacterium tuberculosis escapes phagosomes using ESAT-6 proteins but succumbs to IFN-γ-primed macrophage autophagy. HIV, however, hijacks dendritic cells to infiltrate lymph nodes undetected-a evasion tactic challenging vaccine design. Emerging mRNA vaccines circumvent this by mimicking viral proteins to induce broad-spectrum antibodies.
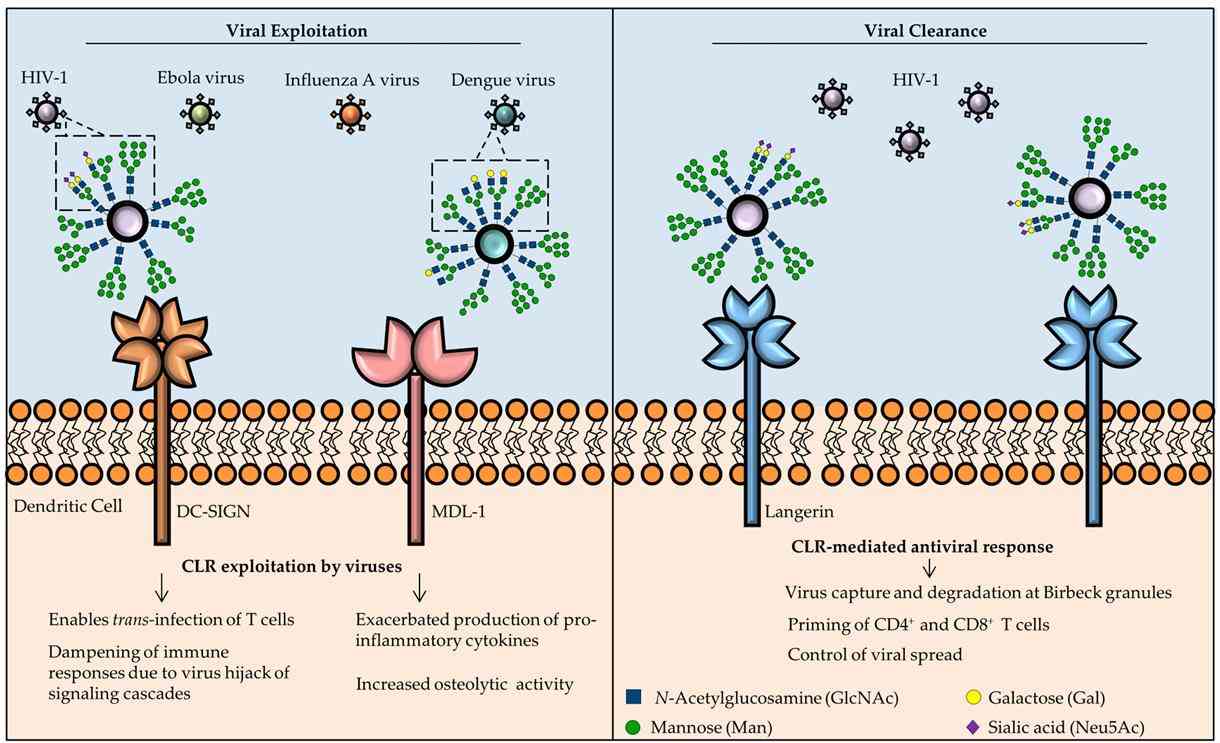 CLRs in antiviral immunity: roles in viral clearance or as targets for viral exploitation. ( Monteiro et al., 2017)
CLRs in antiviral immunity: roles in viral clearance or as targets for viral exploitation. ( Monteiro et al., 2017)
Modern sequencing technologies are playing a pivotal role in respiratory virus detection, including pathogens like influenza viruses and coronaviruses. Tools such as the Respiratory Virus Target Enrichment Panel Kit from CD Genomics enable researchers to simultaneously detect over 40 respiratory viruses-like influenza A/B, respiratory syncytial virus, and adenovirus-even in samples with low viral loads. Utilizing hybrid capture or amplicon sequencing techniques, these advanced methods offer rapid and comprehensive analysis.
Pathogens Challenges & Evasion Tactics
Pathogens excel in evolutionary deception. Influenza utilizes antigenic drift, subtly modifying HA proteins yearly, while antigenic shift-evident in the 2009 H1N1 pandemic-combines animal and human viral gene fragments. HIV leverages error-prone replication to generate endless gp120 variants, evading antibodies like a shape-shifting target.
Bacteria employ molecular mimicry as camouflage. Streptococcus pyogenes produces M protein, which mirrors human cardiac myosin, tricking antibodies into attacking heart tissue-a mechanism driving rheumatic fever. Pseudomonas aeruginosa releases elastase to dismantle complement proteins, erasing immune detection markers like a stealth agent.
Cellular infiltration tactics mirror espionage. Salmonella deploys a type III secretion system to inject SopE into macrophages, hijacking cytoskeletal machinery to create a survival niche. Mycobacterium tuberculosis uses ESAT-6 protein to rupture phagosomes, escaping into the cytoplasm. Viruses manipulate host signaling: Epstein-Barr virus produces BCRF1, a mimic of human IL-10, to suppress Th1 cell activity, while herpesviruses block antigen presentation by disabling TAP transporters with ICP47 proteins.
Summary: How Our Immune System Detects Pathogens
Immune recognition is an evolutionary dialogue spanning millennia. Innate receptors decode ancient pathogen signatures, while adaptive immunity crafts unique solutions through genetic recombination. TLR4's response to LPS not only combats immediate threats but also epigenetically "primes" cells for faster future reactions-a phenomenon termed trained immunity.
On this microscopic battlefield, macrophages anchor defenses like infantry, while neutrophils execute cavalry-like charges. T and B cells mirror modern warfare: precise targeting followed by strategic strikes. Regulatory T cells and complement inhibitors act as safeguards, preventing collateral damage.
Medical advances mirror these principles. From Jenner's smallpox vaccine to mRNA technology, we mimic immune strategies to develop defenses. Cell-based sequencing reveals immune cell diversity, paving the way for personalized therapies-like tailoring vaccines to individual TCR profiles or targeting tumor-associated macrophages.
Ultimately, the immune system teaches that survival hinges not on eliminating all threats, but on dynamic equilibrium. As COVID-19 vaccines demonstrate, training immunity to mitigate harm-rather than eradicate pathogens-may be life's enduring strategy in this ceaseless evolutionary dance.
Learn More:
References:
- Yu Y. "The Function of NK Cells in Tumor Metastasis and NK Cell-Based Immunotherapy." Cancers (Basel). 2023 15(8):2323.https://doi.org/10.3390/cancers15082323.
- Lee CC, Avalos AM, Ploegh HL. "Accessory molecules for Toll-like receptors and their function." Nat Rev Immunol. 2012 12(3):168-179.https://doi.org/10.1038/nri3151.
- Gaffney E, Murphy D, Walsh A, Connolly S, Basdeo SA, Keane J, Phelan JJ. "Defining the role of neutrophils in the lung during infection: Implications for tuberculosis disease." Front Immunol. 2022 13:984293.https://doi.org/10.3389/fimmu.2022.984293.
- Monteiro JT, Lepenies B. "Myeloid C-Type Lectin Receptors in Viral Recognition and Antiviral Immunity." Viruses. 2017 9(3):59.https://doi.org/10.3390/v9030059.
 The human immune system and its functions. (Yu et al., 2023)
The human immune system and its functions. (Yu et al., 2023) Accessory molecules mediate ligand binding and delivery to surface and endosomal TLRs. (Lee et al., 2012)
Accessory molecules mediate ligand binding and delivery to surface and endosomal TLRs. (Lee et al., 2012) The effects of macrophages on neutrophils. (Gaffney et al., 2022)
The effects of macrophages on neutrophils. (Gaffney et al., 2022) CLRs in antiviral immunity: roles in viral clearance or as targets for viral exploitation. ( Monteiro et al., 2017)
CLRs in antiviral immunity: roles in viral clearance or as targets for viral exploitation. ( Monteiro et al., 2017)