MassArray for Virus Detection: Introduction, Features, and Applications
Viruses are entities that can only multiply inside living cells. They hijack cellular machinery to channel resources for their own replication and assembly resulting in exponential colonization of neighbouring tissues. Since the beginning of 2020, COVID-19 has created a huge challenge for communities and laboratories worldwide. Addressing this crisis requires an accurate testing method capable of processing large numbers of samples at a lower price and time consumption. Faster diagnostics could greatly reduce the risk of infection from asymptomatic individuals which usually leads to outbreaks. Testing capacities have been improved since then but with the steady rise of cases, there is still a need for more reliable and cost-effective testing methods such as MassARRAY based on mass spectrometric (MS) techniques. It could complement or totally replace the “gold standard” detection or real-time reverse transcriptase RT-PCR which faces numerous bottlenecks such as a shortage of reagents.
Molecular tests such as RT-PCR and qPCR can only target one gene sequence. Since mutations can happen anywhere in the virus genome, false negatives may arise from the diagnosis using current molecular methods. Recently, the MassARRAY platform based on mass spectrometry using MALDI-TOF (Matrix-Assisted Laser Desorption/Ionization-Time of Flight) was accepted as a diagnostic method for COVID-19 because of its innovative features such as same-day results from DNA to data. As detailed in Figure 1, MALDI-TOF MS distinguishes DNA molecules through ionization resulting in a variable known as the time-of-flight which directly translates to its molecular mass without the need of additional labelling reagents such as fluorescent techniques.
The MassARRAY system could accommodate samples loaded in at most 96 to 384 well plates. It offers higher precision, reproducibility, sensitivity, and cost-effectiveness which could certainly help every laboratory in overcoming hurdles in virus diagnosis during epidemics or pandemics dealing with a large number of samples. When compared with RT-PCR, the MassARRAY platform has the same hands-on time with concordant results. Moreover, it can be customized to analyze research data from viral DNA, RNA, and proteins.
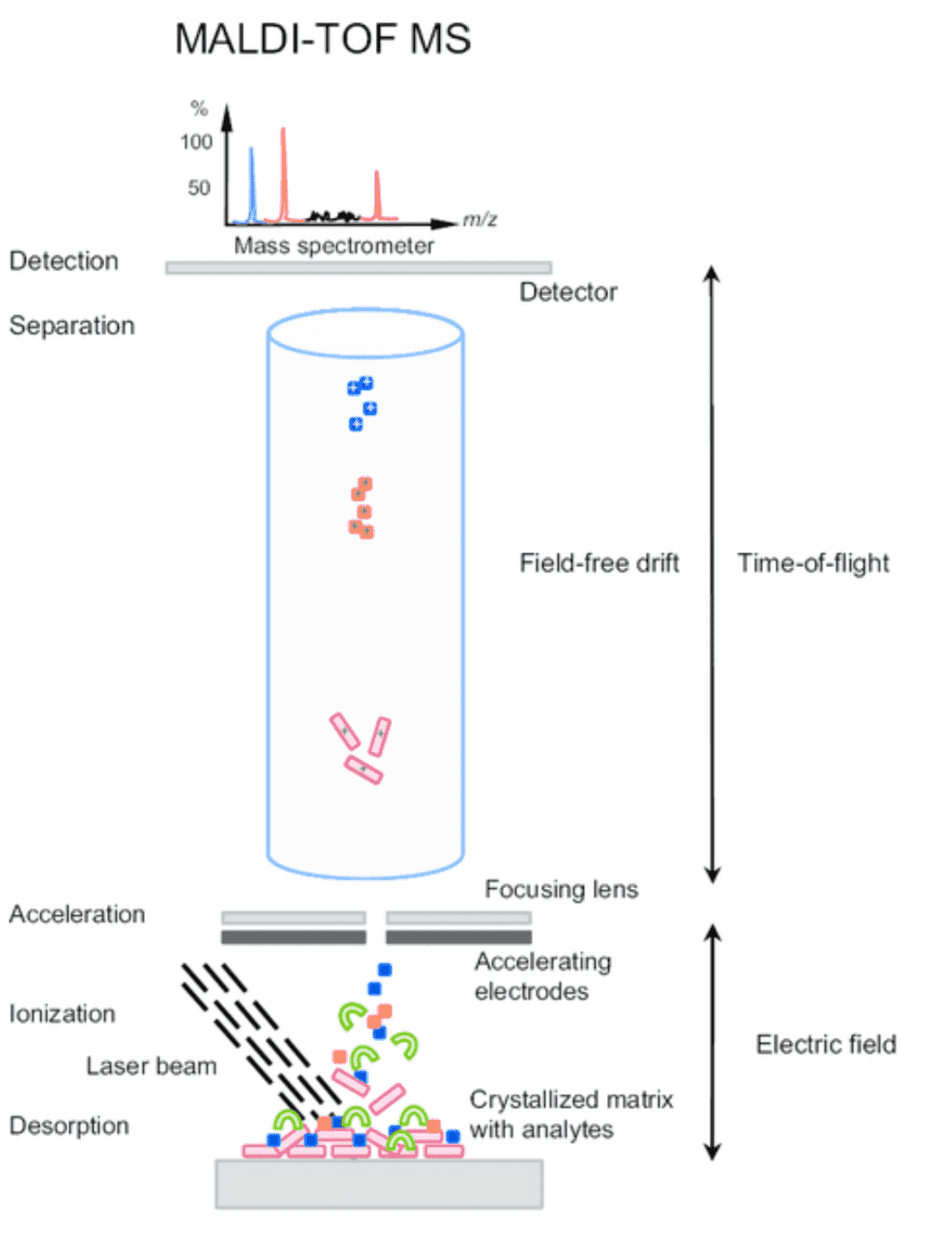 Figure 1. The MALDI-TOF MS Principle (Lavigne, 2013)
Figure 1. The MALDI-TOF MS Principle (Lavigne, 2013)
MassARRAY analysis involves determining which terminator was added to the primer and will assign a call or a nucleotide (A/T/C/G) Based upon the mass of the unextended primer for each assay. If there is no DNA template only the unextended primer will be detected. For suspected SNPs or mutations, mass spectra data were inspected to verify the call whether it is a suspected false-positive result or not.
References:
- Basu P, Chandna P, Bamezai RN, et al. MassARRAY spectrometry is more sensitive than PreTect HPV-Proofer and consensus PCR for type-specific detection of high-risk oncogenic human papillomavirus genotypes in cervical cancer. Journal of clinical microbiology. 2011; 49(10):3537-44.
- Peng J, Yang F, Xiong Z, et al. Sensitive and rapid detection of viruses associated with hand foot and mouth disease using multiplexed MALDI-TOF analysis. Journal of clinical virology. 2013; 56(2):170-4.
- Wandernoth P, Kriegsmann K, Groh-Mohanu C, et al. Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by Mass Spectrometry. Viruses. 2020; 12(8):849.
- Xiu L, Zhang C, Wu Z, et al. Establishment and application of a universal coronavirus screening method using MALDI-TOF mass spectrometry. Frontiers in microbiology. 2017; 9;8:1510.
- Lavigne JP, Espinal P, Dunyach-Remy C, et al. Mass spectrometry: a revolution in clinical microbiology?. Clinical Chemistry and Laboratory Medicine. 2013; 51(2):257-70.
* For research purposes only, not intended for clinical diagnosis, treatment, or individual health assessments.
Related Services
Related Products
 Figure 1. The MALDI-TOF MS Principle (Lavigne, 2013)
Figure 1. The MALDI-TOF MS Principle (Lavigne, 2013)