Rice Genome Sequencing: Key Discoveries and Future Implications
The study of rice genomics has transitioned from reliance on a single reference genome into the pan-genome era. By integrating multi-omics data, AI models, and gene-editing technologies, this advancement is propelling molecular breeding into a precision-driven stage. Future efforts should focus on overcoming bottlenecks such as deciphering complex genomic structures and enhancing functional validation efficiency. Meanwhile, international collaboration is crucial to democratizing technological advancements, thereby providing scientific underpinnings for addressing global food security challenges. This article systematically reviews rice genome sequencing research across four dimensions: historical context, core advancements, applications and breakthroughs, as well as persisting challenges.
Rice Genome Sequencing Background
Rice (Oryza sativa L.) is one of the world's major food crops, serving as the staple food for over 60% of the global population. For a long time, rice cultivation has faced numerous challenges, including increasingly severe pest and disease threats coupled with pesticide overuse, excessive fertilizer application, water scarcity and extreme climate change pressures, as well as the urgent need for environmentally friendly and sustainable agricultural development. These factors collectively underscore the milestone significance of rice genome sequencing research for agricultural science and food security.
In 2002, Japanese scientists first completed the draft genome of japonica rice, followed by the high-quality sequencing of indica rice by an international consortium in 2005, marking the first full genome deciphering of any crop species. This breakthrough revealed the functional and regulatory networks of approximately 40,000 rice genes, laying the foundation for understanding molecular mechanisms underlying critical traits such as photosynthesis, disease resistance, and stress tolerance. The genomic data has significantly accelerated molecular marker-assisted breeding, enabling scientists to precisely edit target genes for high yield, superior quality, and drought/flood resilience. These advancements have driven the development of new varieties like "Green Super Rice," providing crucial technological support to address food security challenges posed by climate change and population growth.
Core Objectives of Rice Genome Sequencing
The core purpose of rice genome sequencing is to comprehensively analyze rice genetic information. By delving into gene structure and function, it aims to identify key genes and loci governing important agronomic traits. This provides a scientific foundation for precision agriculture and molecular breeding.
Building Foundational Databases
Application of Sequencing Technologies: Initially, first-generation sequencing technologies, such as the Sanger method, were used for rice genome sequencing. However, these methods were costly and inefficient. With the emergence of second-generation sequencing technologies (e.g., Illumina sequencing), sequencing throughput was significantly increased, costs were reduced, and rice genome sequencing advanced rapidly. For example, the rice genomics research team at Huazhong Agricultural University utilized the Illumina platform to perform genome resequencing of 1,508 Asian cultivated rice varieties from five continents (China, India, Japan, South Korea, and the United States), generating vast amounts of genomic sequence data.
Sequence Assembly and Refinement: After obtaining large-scale sequencing data, bioinformatics tools are employed for sequence assembly to construct a complete genomic framework. Additionally, sequences require correction and refinement to fill gaps, ensuring accuracy and completeness. This process guarantees the reliability of sequence information stored in the database.
Establishing Gene Functional Annotation and Molecular Marker Maps
Gene Functional Annotation: Multiple bioinformatics approaches are used to annotate gene functions in the rice genome. For instance, Sequence similarity alignment: Rice genes are compared with functionally characterized genes in public databases (e.g., SwissProt) to infer potential functions. Gene structure prediction: Software tools analyze coding regions, introns, exons, and other structural features. Multi-omics integration: Gene expression profiles, protein interaction data, and mutant phenotypes further elucidate gene functions and mechanisms. Systems like RiceGAAS enable automated genome annotation, including structural and functional labeling of genes.
Molecular Marker Development and Map Construction: Abundant molecular markers, such as simple sequence repeats (SSRs) and single nucleotide polymorphisms (SNPs), are identified from rice genome sequences. By scanning genomic variations across cultivars and correlating genotypic data with phenotypes (e.g., using genetic or association populations), high-density molecular marker maps are constructed. For example, Chinese scientists developed genome-wide SNP markers and high-throughput, cost-effective genotyping technologies (e.g., the RICE6K and high-density SNP arrays). These advancements enrich rice genomic databases and provide powerful tools for genetic breeding.
Molecular Breeding
The latest study has constructed the highest-resolution pangenome reference to date for wild and cultivated rice, integrating high-quality genomic data from 145 accessions of Asian cultivated rice and common wild rice. This effort added 3.87 billion new base pairs and identified 69,531 genes, 20% of which are wild rice-specific and closely associated with disease resistance, stress tolerance, and environmental adaptation. Through pangenome analysis, the research team precisely localized 1,184 high-copy disease-resistant loci in wild rice (including two functionally validated blast-resistant genes), highlighting wild rice's role as a critical "genetic reservoir" for molecular breeding. This study established a near-saturation wild rice pangenome database, achieving systematic integration of wild rice genetic resources and effectively bridging the genomic research gap between wild and cultivated rice. Scientists can now leverage this resource to mine superior alleles from wild rice, trace the evolutionary origins of key genes, and decipher mechanisms underlying environmental adaptation and phenotypic plasticity in rice. Amid growing global food security challenges, this work provides essential genetic resources for accelerating rapid de novo domestication and precision breeding of novel rice varieties with enhanced stress tolerance, improved resource-use efficiency, and yield breakthroughs.
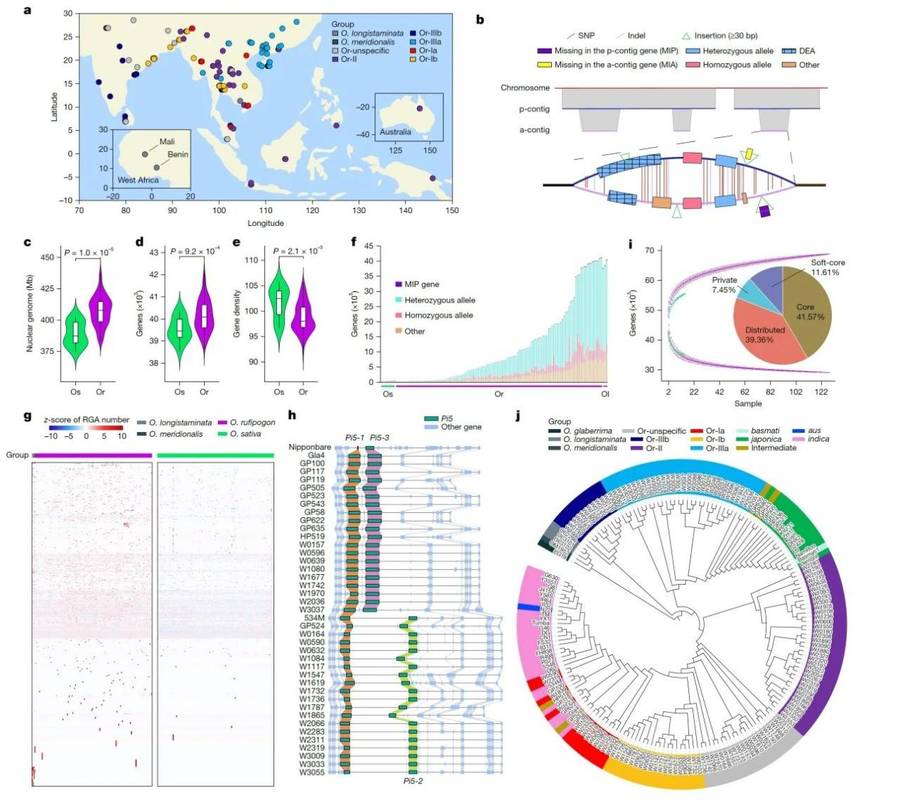 Pan-genome maps of wild rice and cultivated rice (Dongling et al.,2025)
Pan-genome maps of wild rice and cultivated rice (Dongling et al.,2025)
Ensuring Food Security
Rice genome research has significantly enhanced breeding efficiency for disease/pest resistance and stress tolerance traits through resistance gene mining, pan-genome resource integration, and gene editing technologies. The genetic diversity of wild rice and the elucidation of novel gene regulatory networks provide scientific support for addressing climate change and food security challenges. Rice sheath blight (RSB), caused by Rhizoctonia solani, is a major disease affecting global rice production. Genetic resistance to RSB in rice is associated with multiple minor-effect genes, each contributing subtle phenotypic effects, while dominant resistance genes remain unidentified. A GWAS based on SNPs and haplotypes was conducted across 259 rice varieties to evaluate their responses to RSB at three developmental stages: seedling, tillering, and booting. A total of 653 genes were identified as associated with RSB resistance, among which the resistance protein RPM1 (OsRSR1) and a protein kinase domain-containing protein (OsRLCK5) were functionally validated through overexpression and knockdown experiments. Further studies revealed that the coiled-coil (CC) domain of OsRSR1 and OsRLCK5 interact with serine hydroxymethyltransferase 1 (OsSHM1) and glutaredoxin (OsGRX20), respectively. OsSHM1 and OsGRX20 were found to participate in reactive oxygen species (ROS) bursts, enhancing plant antioxidant capacity. This research uncovered a regulatory model of RSB resistance mediated by the glutathione (GSH)-ascorbate (AsA) antioxidant system. These findings deepen our understanding of RSB resistance mechanisms and provide superior genetic resources for disease-resistant rice breeding.
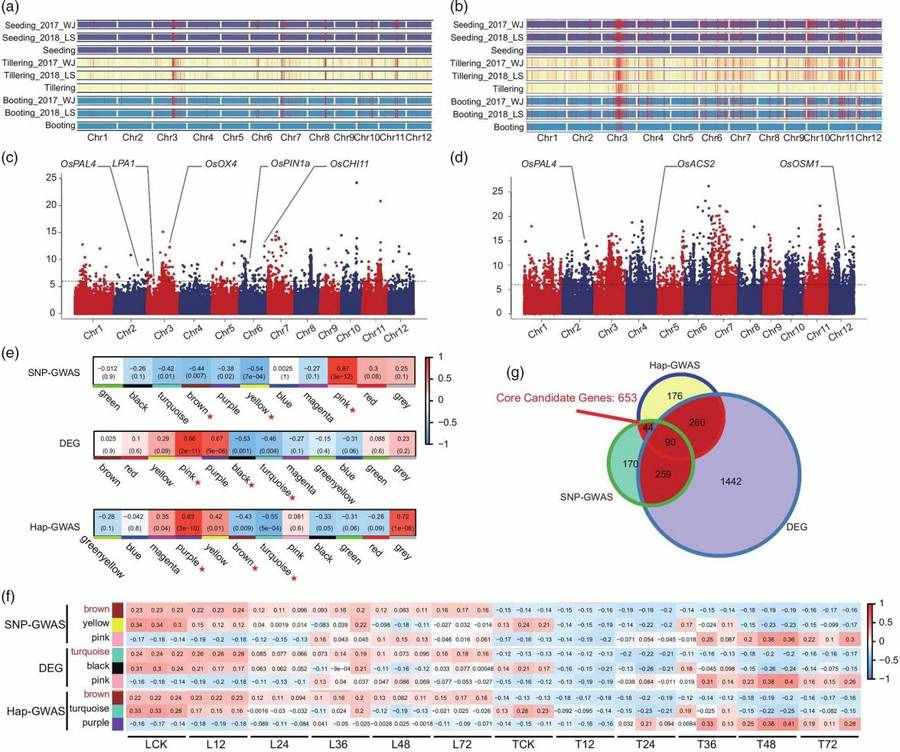 GWAS and weighted WGCNA of rice accessions for identification of RSB resistance candidate genes (Wang A et al.,2021)
GWAS and weighted WGCNA of rice accessions for identification of RSB resistance candidate genes (Wang A et al.,2021)
Applications of Genome Sequencing
Rice genome sequencing, a critical breakthrough in modern agricultural technology, connects deep gene resource exploration with innovative breeding practices. By analyzing rice genome structure and function, scientists can identify key genetic loci for disease resistance, stress tolerance, and high yield, providing precise targets for molecular marker development. Integrating high-throughput sequencing with multi-omics analysis, researchers can create high-precision genetic maps and validate gene functions quickly via genome-wide association analysis and gene-editing technologies. This overcomes the traditional breeding barrier of negative correlation between disease resistance and yield.
Precision Breeding Technologies
Marker-assisted selection (MAS) leverages molecular markers closely linked to target trait genotypes for assisted selection of specific genes. It is reliable, efficient, ensures durable disease resistance, and offers abundant loci. MAS precisely selects target traits, boosting breeding efficiency without environmental influence, thus accelerating the breeding process. Rice genome sequencing provides a comprehensive genetic information foundation for MAS, enhancing breeding efficiency and precision. Studies have integrated MAS with other technologies to introduce the broad-spectrum, efficient rice blast resistance gene Pi2 into superior rice varieties lacking this gene. This has successfully developed super rice varieties that are resistant to rice blast while maintaining or even improving yield and quality, overcoming the negative correlation between yield, quality, and disease resistance in crop breeding, and offering new ideas for rice breeding.
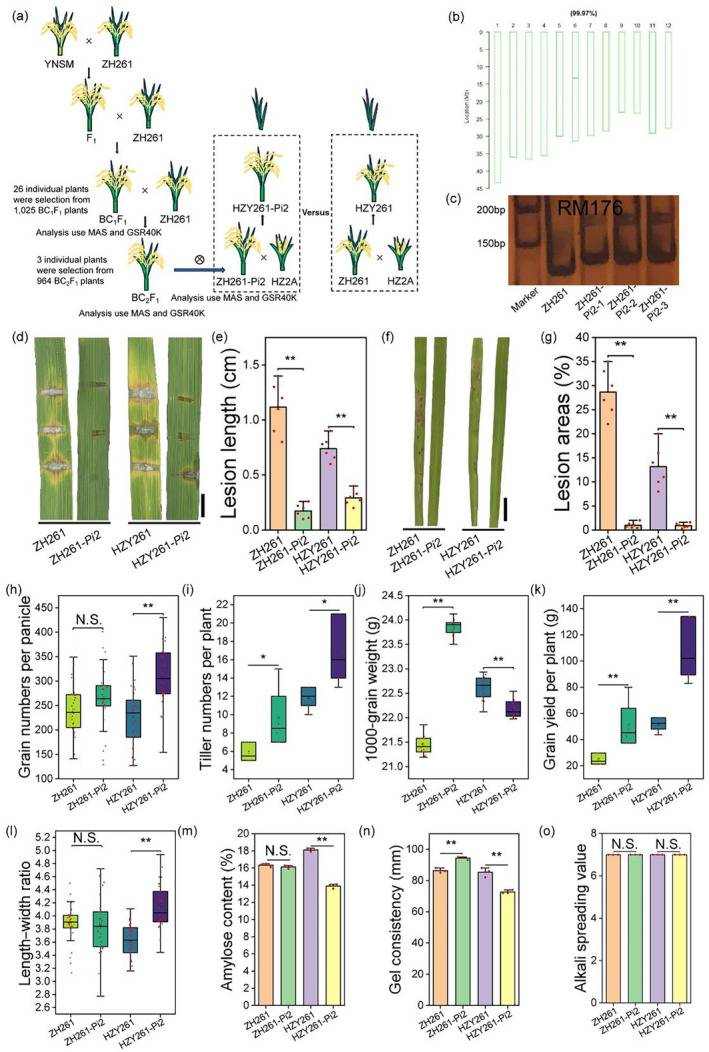 Generation of a novel super rice for blast resistance by integrating MAS and GSR40K detection.(Guo Naihui et al.,2021)
Generation of a novel super rice for blast resistance by integrating MAS and GSR40K detection.(Guo Naihui et al.,2021)
Functional Genomics Research
GWAS (Genome-Wide Association Study), as a critical tool for dissecting the genetic basis of complex traits, plays a pivotal role in rice molecular breeding. By integrating large-scale germplasm resources, multi-dimensional phenotypic data, and advanced algorithms, GWAS enables efficient identification of genetic loci associated with yield, stress resistance, quality, and other traits, driving the development of molecular markers, functional validation of genes, and precision breeding design.
In one study, a systematic evaluation of 3,606 rice accessions generated 212 phenotypic datasets. Large-scale GWAS based on 212 environmental datasets identified 3,131 QTLs (quantitative trait loci), of which 450 QTLs were stable across at least two environments, 125 exhibited pleiotropic effects, and 2,642 QTLs showed strong associations only in a single location/year. Grain shape, a fundamental trait determining rice yield and quality, is of great significance for varietal improvement. The authors identified a primary peak on chromosome 3, where the lead SNP (Chr03:35,155,927) was significantly associated with grain length. This SNP resides within a 60-kb genomic block containing 14 genes, among which OsGL3.6 was identified as the most likely causal gene. Haplotype analysis revealed three major haplotypes of OsGL3.6 in 3,547 accessions, further confirming its role in regulating grain length. CRISPR knockout of the candidate gene OsGL3.6 (an IAA hydrolase gene) resulted in significantly shortened grain length, functionally validating its regulatory role.
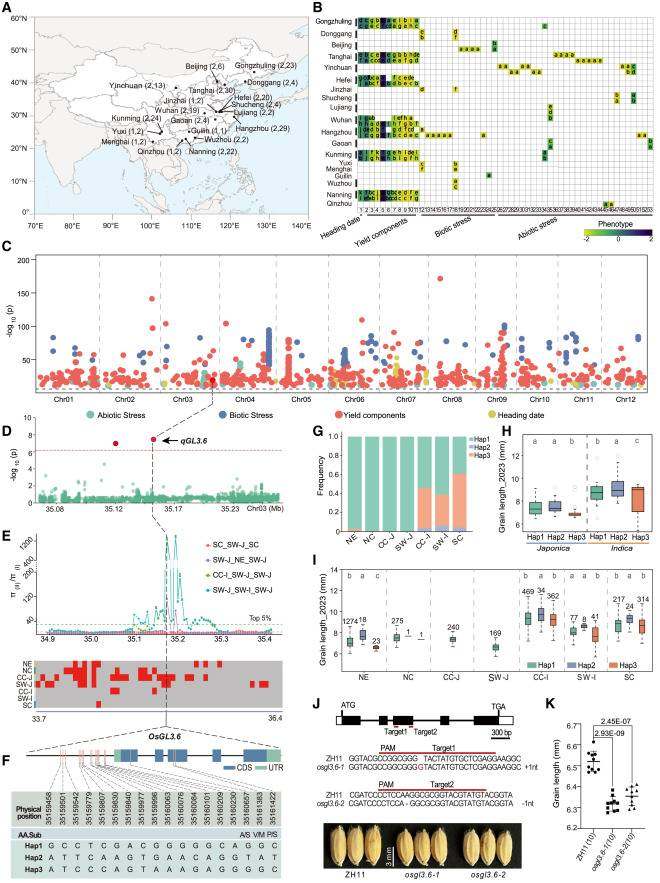 Large-scale GWAS and identification of OsGL3.6 through integration of GWAS results with selective sweep and IBD analyses.(Ma X et al.,2025)
Large-scale GWAS and identification of OsGL3.6 through integration of GWAS results with selective sweep and IBD analyses.(Ma X et al.,2025)
Services you may interested in
Challenges and Future Directions
In recent years, significant progress has been made in rice genome sequencing. However, several technical bottlenecks remain, including challenges in complex structural assembly, functional annotation, integration of genetic diversity, high-throughput validation, and cross-species applications. To overcome these limitations, future efforts must integrate long-read sequencing technologies, AI-driven models (e.g., Osei), and pan-genome studies. These advancements will provide more comprehensive genomic resources to support precision breeding and enhance global food security.
Current Technical Bottlenecks
Challenges in Assembling Complex Structural Regions: Repetitive regions of the rice genome—including telomeres, centromeres, ribosomal DNA, and transposable elements—remain difficult to fully assemble using traditional sequencing due to their high repetitiveness and structural complexity. For example, the widely used Nipponbare reference genome retained ~3% unresolved gaps despite iterative updates, and a telomere-to-telomere gap-free assembly was only achieved in 2023 using next-generation sequencing. Assembly failures in these regions can lead to gene annotation errors, such as corrected structural mistakes and the addition of 1,324 novel protein-coding genes.
Functional Annotation and Regulatory Element Analysis: Deciphering the roles of regulatory elements (e.g., promoters, enhancers) and their links to agronomic traits remains challenging. While the deep learning model Osei predicts regulatory sequences using chromatin features, it requires 8.5 million regulatory peak entries for training. Additionally, functional mechanisms of stress- and disease-resistant genes in wild rice (e.g., blast-resistant genes) remain unvalidated despite their identification in pan-genome studies.
Genetic Diversity and Pan-Genome Complexity: A single reference genome fails to capture rice's full genetic diversity, leading to the loss of critical genes (e.g., stress-resistance genes) during domestication. Asian cultivated rice exhibits significantly lower diversity than wild rice, necessitating pan-genome studies (e.g., a super pan-genome of 251 accessions) to recover lost genetic resources. Technical barriers, such as high-cost genotyping and haplotype analysis, further hinder studies on wild-cultivated rice gene flow.
Limitations in High-Throughput Functional Validation: Protein-protein interaction studies rely on low-throughput methods like yeast two-hybrid. Although BIP-seq improves throughput, its accuracy remains suboptimal (~62.5%). Developing high-precision molecular markers (e.g., SNPs) faces challenges like high costs and lengthy validation cycles.
Data Integration and Cross-Species Limitations: The cross-species applicability of genomic tools is limited. For example, the Osei model performs well in maize and Arabidopsis but requires broader validation. Integrated analysis of multi-omics data (genome, epigenome, transcriptome) lacks standardized tools, hindering systematic gene function discovery.
Global Collaboration Prospects
The global collaboration prospects for rice genome sequencing are highly promising, centered on integrating resources, sharing data, and advancing technology accessibility through multinational cooperation. International efforts have established global genetic resource repositories (e.g., the International Rice Research Institute completed sequencing 3,000 core germplasms with multinational partners) and pan-genome studies that uncover how genetic variations influence agronomic traits. Technologically, high-throughput sequencers have reduced per-sample costs to below $100, while cloud computing platforms enable scientists worldwide to collaboratively analyze massive datasets. In practical applications, perennial rice technology—piloted in Africa via the Belt and Road Initiative—achieves an annual yield of three harvests at 521 kg per mu (equivalent to ~3.2 tons/hectare), and genome-guided breeding has shortened development cycles from 12 years to 3 years. Standardization initiatives and talent development programs reduce technical barriers, while stress-resistant cultivars (e.g., Green Super Rice) and equitable technology transfer help address climate change and food security. Moving forward, deepening technology democratization, data openness, localized applications, and policy coordination will accelerate the transition from lab to field, providing foundational support for global sustainable agriculture.
Conclusion
Genome sequencing is like a magical key that has unlocked the genetic mysteries of rice. It has revolutionised rice research, enabling scientists to precisely locate, identify, and analyse every rice gene and its function. This gives deep insight into rice growth, development, disease and pest resistance, and quality formation. It has sped up the breeding of good rice varieties and offered rich genetic resources for agriculture. Despite a growing global population, rising food demand, and climate change challenges, this technology helps researchers find potential high - yield and stress - resistant genes in rice. Using these genes in breeding improves rice varieties, creating high - yield, high - quality rice that adapts to different environments. This stabilises food production.
References
- Dongling, Guo,Yan, Li,Hengyun, Lu et al. "A pangenome reference of wild and cultivated rice" .Nature, 2025, 0: 0. https://doi.org/10.1038/s41586-025-08883-6
- Wang A , Shu X , Jing X ,et al."Identification of rice (Oryza sativa L.) genes involved in sheath blight resistance via a genome-wide association study" .Plant Biotechnology Journal, 2021. https://doi.org/10.1111/pbi.13569
- Guo Naihui, An Ruihu, Ren Zongliang et al. "Developing super rice varieties resistant to rice blast with enhanced yield and improved quality". Plant Biotechnol, 2025, 23: 232-234. https://doi.org/10.1111/pbi.14492
- Ma X , Wang H , Yan S ,et al."Large-scale genomic and phenomic analyses of modern cultivars empower future rice breeding design" .Molecular Plant, 2025, 04, 27 https://doi.org/10.1016/j.molp.2025.03.007
 Send a Message
Send a MessageFor any general inquiries, please fill out the form below.