ONT Direct RNA Sequencing: Translational Applications in Biomedical Research
As research into RNA modification has progressed, the capacity to detect chemical modifications in RNA molecules has emerged as a significant instrument in the comprehension of gene regulation. Conventional sequencing methodologies, including MeRIP-seq, depend on antibody enrichment and are characterised by limitations such as low resolution and the loss of modifications. In contrast, Oxford Nanopore Technologies (ONT) Direct RNA Sequencing (DRS) has been shown to be capable of reading RNA molecules directly through nanopores to detect modifications at the single-molecule and single-base level. This has been demonstrated to improve the resolution of RNA modifications, as well as to reveal the structural features of transcripts, thus expanding the boundaries of RNA biology research
Compared with traditional indirect methods, ONT DRS can directly capture intact RNA molecules without the need for fragmentation or antibody enrichment, and accurately identify modifications such as m6A, m5C, pseudouridine, etc., as well as resolving key information such as shear variants and polyadenylate tail length.
In terms of functional research, ONT DRS has clear advantages in the identification of long non-coding RNA (lncRNA) modifications, and is able to accurately resolve individual m6A sites and analyse their modification frequencies, thus revealing the dynamic distribution patterns of RNA modifications. Its high-resolution epitranscriptomic fingerprinting technology has shown great potential in cancer diagnosis, disease mechanism analysis and RNA therapeutic strategy development. For instance, specific rRNA modification patterns have been found to be closely related to tissue specificity and developmental stage, and can be used for classification and diagnosis of diseases such as lung cancer. Furthermore, DRS technology has made breakthroughs in the regulation of liver metabolism, bone tissue formation, and non-coding RNA function analysis, providing new ideas for precision medicine and RNA therapy.
Technological Evolution of ONT DRS
As research into RNA modification continues to progress, the accurate detection of chemical modifications on RNA molecules has become imperative in order to facilitate a more profound comprehension of gene regulation. Conventional sequencing methodologies, such as MeRIP-seq, are contingent on the use of antibodies for the enrichment of target modifications and are susceptible to limitations including, but not limited to, diminished resolution and the loss of modifications. The advent of nanopore sequencing technology has enabled the transition from indirect speculation to direct sequencing of RNA modifications at the single-molecule, single-base level. ONT DRS has been shown to not only accurately resolve RNA modifications, but also to comprehensively characterise the structural features of transcripts, thereby greatly expanding the boundaries of RNA biology research.
From Indirect Inference to Direct RNA Capture
The conventional MeRIP-seq technique is dependent on the use of antibodies for the enrichment of m6A-modified RNA fragments, and its resolution is constrained by the size of the fragments (100-200 nt), which makes it challenging to accurately locate individual modification sites. ONT DRS, conversely, utilises nanopores to directly read raw RNA molecules without the need for fragmentation or antibody enrichment. In addition to detecting modifications such as m6A, m5C, and pseudouridine, DRS also enables comprehensive resolution of features pertaining to transcript isoforms, splice variants, and Poly(A) tail lengths at the single-base level. Furthermore, Direct RNA sequencing is characterised by ease of operation and a reduction in experimental time to one third of MeRIP-seq, while maintaining high levels of reproducibility and cost controllability. This development represents a significant advancement in RNA methylation research, ushering it into a new era of single-base direct sequencing.
Core Analytical Modules and Innovations
The m6A modification prediction by Direct RNA sequencing exhibited a high degree of consistency with MeRIP-seq and m6A-REF-seq, with validation rates of 81% and 80%, respectively.In comparison with MeRIP-seq, which exclusively enriches m6A peaks at the 3' end, Direct RNA sequencing possesses the capability to accurately identify individual m6A sites on full-length transcripts and to analyse the modification frequency, thereby unveiling the dynamic distribution pattern of m6A modifications. In the context of lncRNA research, Direct RNA sequencing has been shown to identify a significantly higher number of m6A sites when compared with MeRIP-seq, thereby highlighting its unique advantage in revealing the fine regulation of RNA modification. This technological breakthrough offers enhanced precision and broader application prospects for RNA methylation studies.
Service you may intersted in
Learn More:
Translational Applications of ONT DRS
Recent advancements in epitranscriptomics research have led to significant progress in our understanding of the function of RNA modifications in gene expression regulation. These developments have also contributed to the advancement of the field in terms of analysis of disease mechanisms and clinical applications. The analysis of RNA modification patterns at various spatial and temporal scales, as well as the study of its role in liver metabolism, bone tissue formation and the functional analysis of non-coding RNAs, is a testament to the broad applications of DRS technology. In particular, in the domains of disease diagnosis, RNA therapeutic strategy development and host-pathogen interaction studies, the analysis of RNA modification information provides a new dimension for precision medicine.In the future, with the advancement of single-cell DRS technology and the deep integration of multi-omics data, epitranscriptomics is expected to become an important driving force for precision medicine and RNA therapy innovation.
Epitranscriptome Fingerprinting
Epitranscriptome fingerprinting is a systematic analytical approach that examines the regulatory networks of gene expression and their central roles in diseases. This method integrates the modification dynamics of various RNA species, including mRNAs, lncRNAs, tRNAs, and other non-coding RNAs. Notably, ONT DRS technology possesses unbiased analytical capabilities and generates high-resolution modification profiles. This technological advancement surpasses the limitations of conventional methods in terms of the analysis of individual RNA types. Consequently, it offers a novel perspective for elucidating cross-scale biological mechanisms. The clinical translation potential of diagnostic models based on multi-category RNA modification fingerprints has been demonstrated, and the deep integration of single-cell DRS technology and functional annotation will promote precision medicine to the 'RNA modification-centric' paradigm.
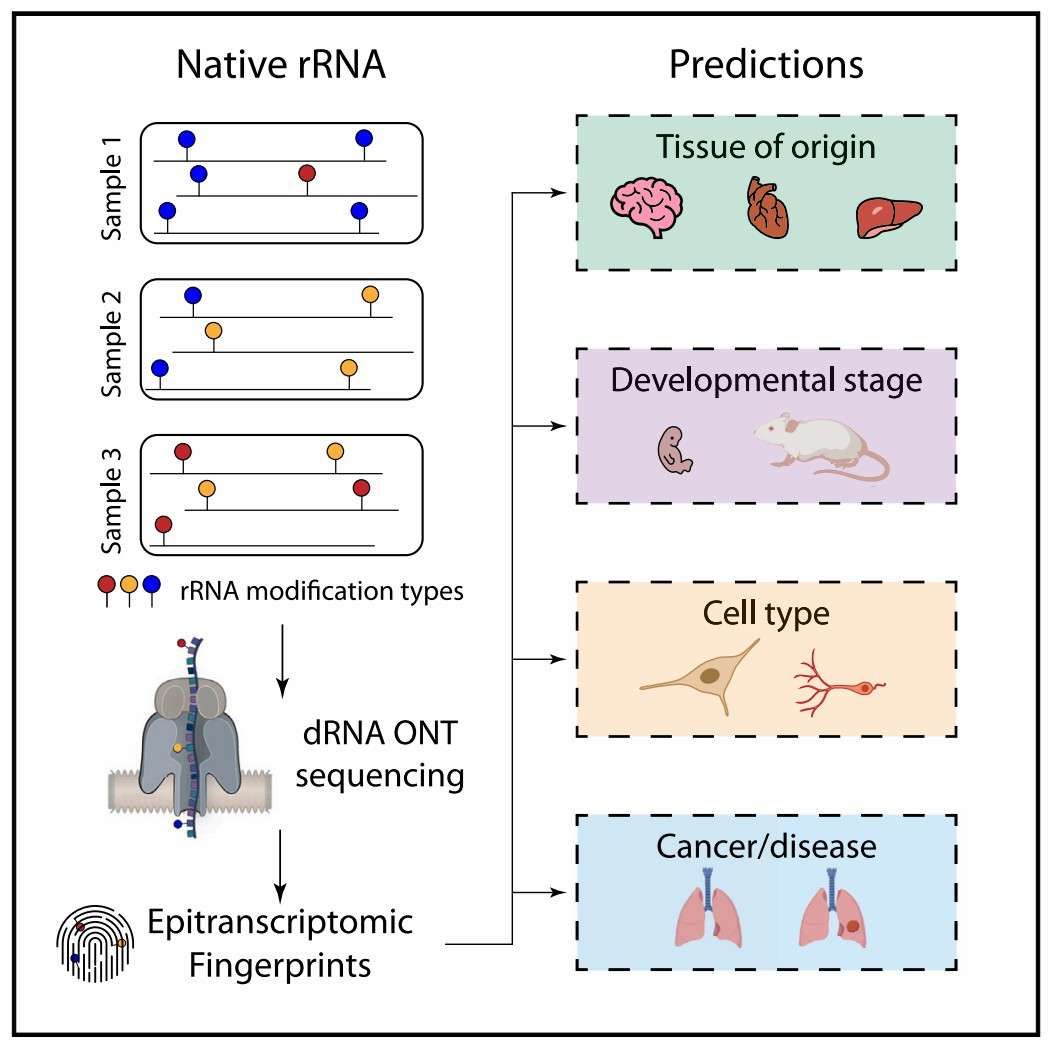 Epitranscriptomic rRNA fingerprinting reveals tissue-of-origin and tumor-specific signatures (Milenkovic et al., 2025)
Epitranscriptomic rRNA fingerprinting reveals tissue-of-origin and tumor-specific signatures (Milenkovic et al., 2025)
A team of researchers from the Barcelona Institute of Science and Technology has recently achieved a significant milestone in the field of mammalian rRNA modification research. Utilising the advanced ONT DRS technique, they have systematically unravelled the intricate temporal and spatial dynamics of these modifications for the first time. The analysis of mouse and human samples across tissues, developmental stages and cancer samples revealed 31 differential modification sites (DMs), 14 of which were newly discovered (e.g. 18S:Ψ890, 28S:Ψ1500) and confirmed that the modification patterns are highly tissue-specific and developmental stage-dependent. For instance, the adult brain displays a distinct modification profile, and the methylation level of 18S:Um355 positively correlates with the degree of cell differentiation, suggesting that it serves as a biomarker of proliferative potential. The technology accurately identifies modifications through the 'base calling error' model, and combined with the random forest model, it can achieve tissue classification in only 100 read lengths (>95% accuracy), which validates the biological robustness of epitranscriptomic fingerprinting. In the context of lung cancer diagnosis, a classification model based on the top 20 DM loci required a mere 250 read lengths to differentiate between normal and tumour tissues (AUC=0.97), thereby significantly outperforming the contribution of traditional Nm modification loci. Furthermore, it was observed that cancer samples exhibited a tendency towards low modification, with 18S:Ψ1136 being significantly downregulated in tumours. The diagnostic value of unannotated modification sites was found to exceed that of known sites, suggesting a potential for novel insights into the development of non-invasive liquid biopsy techniques.The impact of tumour cell content on assay sensitivity requires further exploration, extending this research to encompass additional cancer subtypes. The findings, published in Molecular Cell, signify a pivotal advancement in the transition of epitranscriptomics from mechanism-based research to clinical application, thereby unveiling a novel dimension for precision medicine grounded on ribosomal heterogeneity.
Disease Mechanism Decoding
The high-resolution nature of DRS technology provides a unique perspective for the analysis of complex disease mechanisms. In the study of liver metabolism, the physiological responses and gene regulation information of the human liver are difficult to obtain directly, and usually rely on animal models for the study. However, significant differences exist between mice and humans at the level of gene regulation, affecting the extrapolation of research results. A recent study in the Journal of Hepatology (2023) utilised DRS to map the human liver transcriptome and epitranscriptome by employing a humanised liver mouse model. This study revealed numerous unannotated transcripts and their roles in metabolic regulation. The study demonstrated that the human liver transcriptome undergoes dynamic changes in response to varying dietary status and transcription factor agonist treatment conditions. It was observed that conventional RNA sequencing methods fail to capture more than 50% of these transcripts. The study further elucidates the complex regulatory network of liver metabolism by demonstrating the impact of changes in poly(A) tail length on gene expression. This study provides new perspectives for understanding the development of human liver diseases.
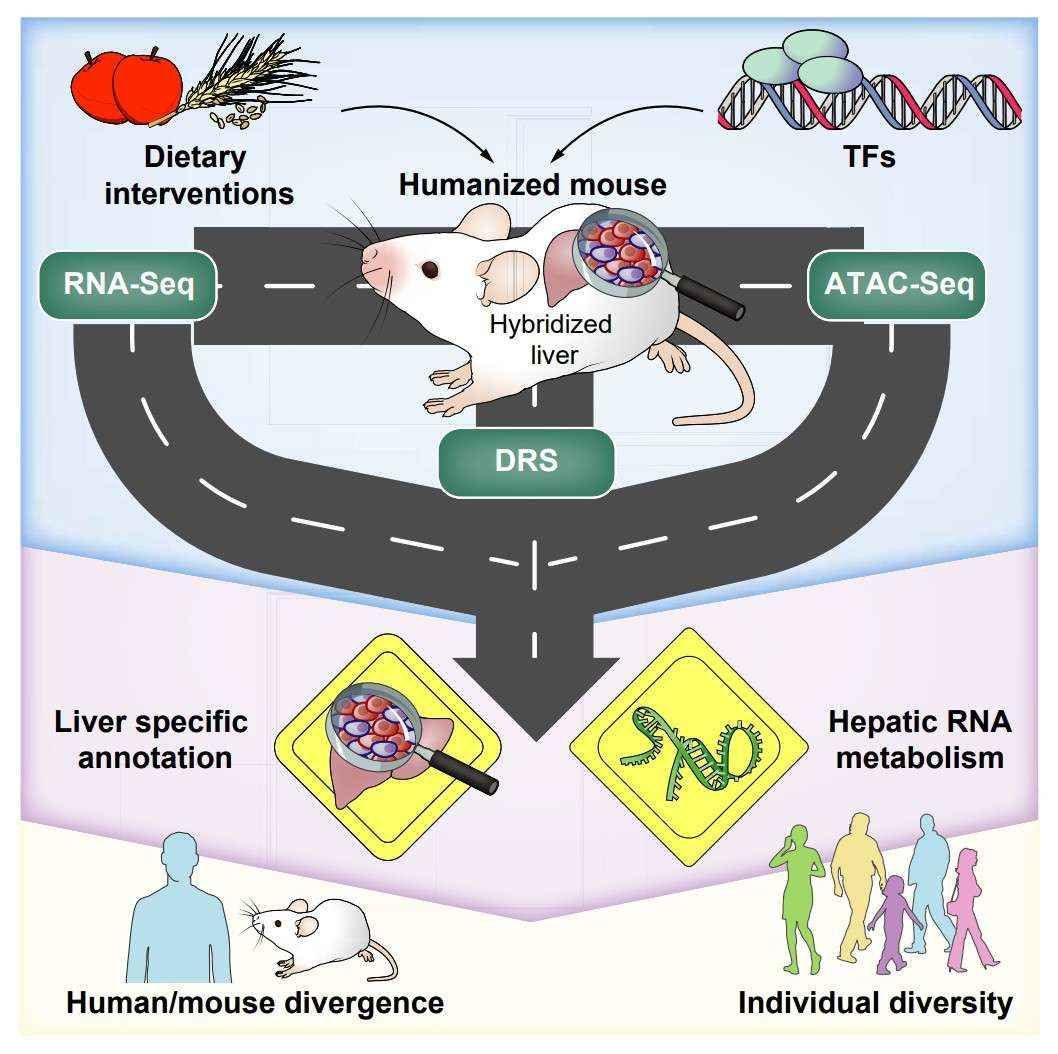 Comprehensive gene profiling of the metabolic landscape of humanized livers in mice (Jiang et al., 2024)
Comprehensive gene profiling of the metabolic landscape of humanized livers in mice (Jiang et al., 2024)
In the domain of bone formation, the process of bone tissue formation is contingent on the accurate expression and modification of collagen, wherein polyadenylation (poly(A) tail adduct) exerts a pivotal function in the stability and translational regulation of mRNA.A study published in Cell Reports (2021) elucidates the pivotal regulatory role of TENT5A, an atypical poly(A) polymerase, in collagen synthesis utilising DRS technology. The study found that TENT5A was able to specifically regulate the poly(A) tail length of COL1A1 and COL1A2 mRNAs, thereby affecting their translation efficiency. Functional experiments further showed that TENT5A knockdown leads to impaired collagen synthesis, which in turn affects bone formation. This study not only reveals the role of TENT5A in bone tissue homeostasis, but also provides new targets for the treatment of diseases such as osteoporosis.
Non-Coding RNA and Therapeutic Development
The modification dynamics and functional regulation of non-coding RNAs represent a growing focus within the field of precision medicine. In a recent publication in Mol Ther Nucleic Acids (2023), the regulatory mechanism of m6A modification on the activity of SINEUP RNA was elucidated through the utilisation of DRS technology. The study revealed that the absence of m6A modification, consequent to METTL3 knockdown, resulted in a significant reduction in the translation enhancement effect of SINEUP RNA. This finding offers a novel direction for the design of gene therapy vectors.
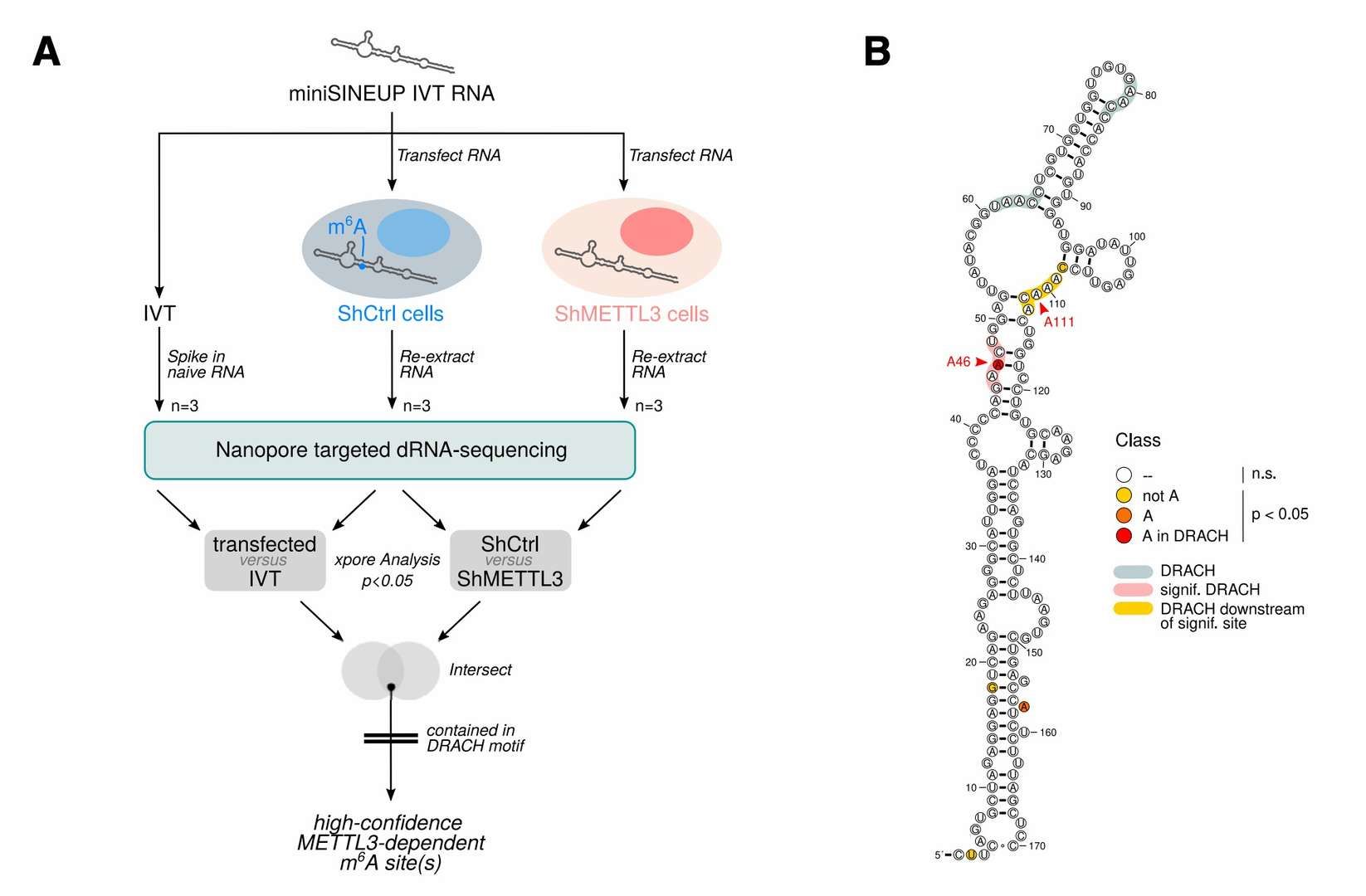 Identification of m6A modification sites in the SINEUP gene (Pierattini et al., 2023)
Identification of m6A modification sites in the SINEUP gene (Pierattini et al., 2023)
The accurate identification of RNA shear variants is imperative for functional genomics and disease research. However, conventional RT-PCR and short-read-length RNA sequencing methods have been shown to introduce artefacts in the detection of shear variants, thereby affecting the accuracy of the data (see Genome Biology, 2021). A comparative analysis of different sequencing methods was conducted using DRS, which revealed that RT-PCR may mislead the identification of shear variants in some cases. By directly sequencing RNA molecules, the researchers identified deviations in the shear patterns of certain transcripts from those predicted by conventional methods. This finding underscores the distinct value of DRS in the realm of shear variant research and provides a novel strategy for the precise detection of RNA shear variants, thereby promoting the advancement of RNA diagnostic technology.
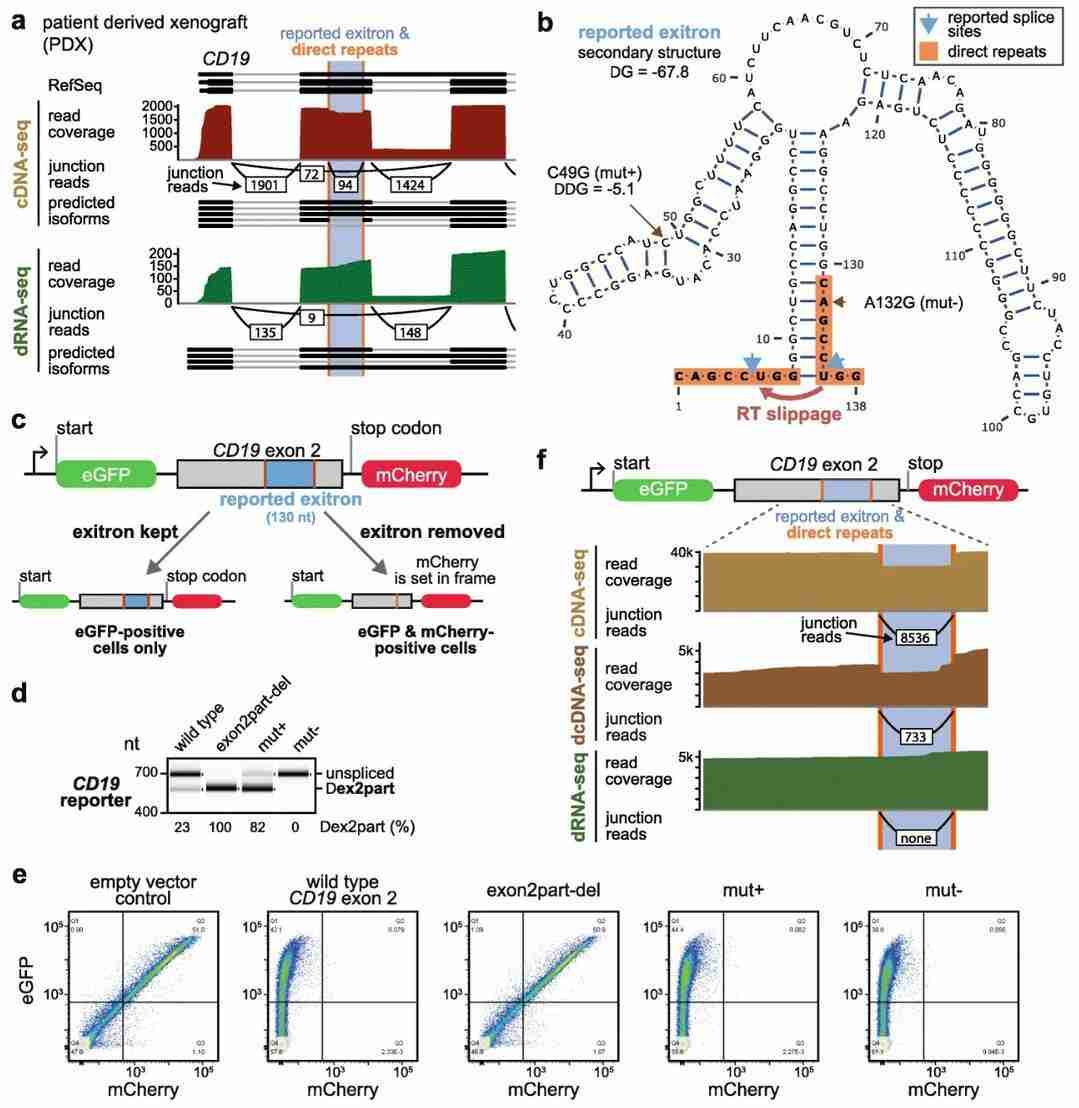 The reported exitron in the CD19 exon 2 is a reverse transcription artifact (Schulz et al., 2021)
The reported exitron in the CD19 exon 2 is a reverse transcription artifact (Schulz et al., 2021)
Immune Regulation and Host-Pathogen Interactions
Innate immunity constitutes the body's primary line of defence against pathogen invasion, and the regulation of poly(A) tail length in mRNAs plays a pivotal role in this process. A study in Science Advances (2022) analysed the role of TENT5, a cytoplasmic poly(A) polymerase, in innate immunity by DRS. The study found that TENT5 promotes immune response by lengthening the poly(A) tails of certain effector mRNAs, enhancing their stability and translational efficiency. In TENT5-mutated model organisms (e.g., Cryptobacterium hidradii nematodes and mice), immune cells showed a significant reduction in their ability to respond to pathogens, suggesting a critical role for TENT5 in host defence. This study not only reveals the link between poly(A) tail modification and immune regulation, but also provides new potential targets for intervention in inflammatory and infectious diseases.
Future Directions and Challenges for ONT DRS
Notwithstanding the distinctive benefits of ONT DRS technology in the context of direct RNA sequencing, the technology is confronted with challenges including restricted read lengths and suboptimal base calling accuracy, particularly in the context of resolving intricate RNA secondary structures. This necessitates further optimisation of nanopore chemistry and algorithms to enhance sequencing precision and resolution. Moreover, the integration of AI-driven computational tools will significantly expedite the development of ONT DRS for automated annotation of RNA modifications and effective integration with multi-omics data. By training deep learning models to identify transcriptome features in different biological environments, a more efficient RNA sequencing and analysis system is expected to be constructed in the future, providing stronger technical support for epitranscriptome research.
However, further validation and standardisation of ONT DRS is required for clinical translation, particularly in the domains of early cancer screening and neurodegenerative disease monitoring, where it demonstrates considerable potential for RNA modification biomarker discovery. Additionally, DRS possesses the capability to discern RNA isoform and modification information in real time, thus providing novel insights into RNA therapeutics, such as the optimisation of mRNA vaccines and antisense oligonucleotide therapy. The establishment of RNA modification detection and isoform analysis protocols that meet clinical standards and are accompanied by a strict quality control system will be key to driving ONT DRS into the field of precision medicine.
References
- Milenkovic, Ivan et al. "Epitranscriptomic rRNA fingerprinting reveals tissue-of-origin and tumor-specific signatures." Molecular cell. 85,1 (2025): 177-190.e7. doi:10.1016/j.molcel.2024.11.014
- Jiang, Chengfei et al. "Comprehensive gene profiling of the metabolic landscape of humanized livers in mice." Journal of hepatology. 80,4 (2024): 622-633. doi:10.1016/j.jhep.2023.11.020
- Pierattini, Bianca et al. "SINEUP non-coding RNA activity depends on specific N6-methyladenosine nucleotides." Molecular therapy. Nucleic acids. 32 402-414. 7 Apr. 2023, doi:10.1016/j.omtn.2023.04.002
- Schulz, Laura et al. "Direct long-read RNA sequencing identifies a subset of questionable exitrons likely arising from reverse transcription artifacts." Genome biology. 22,1 190. 28 Jun. 2021, doi:10.1186/s13059-021-02411-1