How to Extract cfDNA
Cell-free DNA (cfDNA) extraction represents a critical first step in liquid biopsy applications, with methodological choices significantly impacting downstream analytical results. This protocol-focused review examines established techniques for isolating cfDNA from biological fluids, with particular attention to pre-analytical variables, extraction efficiency, and quality control measures relevant to clinical diagnostics and research applications.
Materials and Reagents
Before diving into the detailed protocols and methodologies for chromatin structure modeling, it is crucial to ensure that all necessary materials and reagents are prepared and readily available. Proper selection and handling of these materials are fundamental to the success of subsequent experiments and analyses. In this section, we will outline the essential components required, including specialized blood collection tubes, centrifuges, DNA extraction kits, and other critical reagents. These materials form the backbone of our experimental setup and lay the foundation for accurate and reliable results.
Essential materials include:
1) EDTA or specialized blood collection tubes (Streck, PAXgene)
2) Refrigerated centrifuge capable of 16,000 × g
3) DNA extraction kits (silica membrane or magnetic bead-based)
4) Nuclease-free water and plasticware
5) Fluorometric quantification reagents (Qubit HS DNA assay)
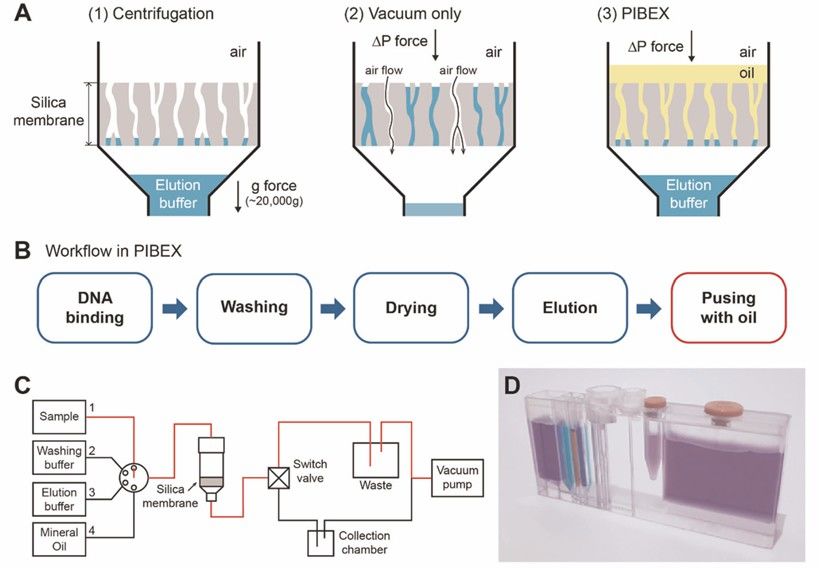 Figure 1. Integrated Microfluidics (PIBEX) for cfDNA extraction.(Hoyoon Lee, et al., 2020)
Figure 1. Integrated Microfluidics (PIBEX) for cfDNA extraction.(Hoyoon Lee, et al., 2020)
Step-by-Step Protocol
Sample Collection and Processing
With the essential materials and reagents in place, the next critical step in our experimental workflow is sample collection and processing. Proper handling of biological samples is vital to ensure the integrity and quality of the data obtained in downstream analyses. In this section, we will detail the procedures for collecting whole blood using appropriate stabilization tubes and outline the specific processing steps, including sequential centrifugation and storage conditions. Adhering to these protocols ensures that the samples are preserved optimally, minimizing degradation and maximizing the yield of high-quality DNA for subsequent chromatin structure studies.
We can proceed with the experiment according to the following procedure:
1) Collect whole blood in appropriate stabilization tubes
2) Process within 2 hours (EDTA) or 7 days (Streck tubes)
3) Perform sequential centrifugation:
1,600 × g for 10 minutes at 4°C
Transfer supernatant to a new tube
16,000 × g for 10 minutes at 4°C
4) Aliquot plasma and store at -80°C if not processing immediately
DNA Extraction
Having successfully collected and processed the samples, the next crucial step in our experimental protocol is DNA extraction. Efficient and accurate extraction of high-quality DNA is essential for downstream applications, including chromatin structure analysis. In this section, we will detail two widely used DNA extraction methods: the silica membrane method and the magnetic bead method. Both approaches have their unique advantages and are suitable for different experimental setups. Additionally, we will outline the critical steps for quality assessment to ensure that the extracted DNA meets the required standards for subsequent analyses. By following these detailed protocols, researchers can obtain reliable and high-quality DNA samples for further study.
We can proceed with the experiment according to the following procedure:
Silica Membrane Method
1) Add proteinase K and lysis buffer to plasma
2) Bind DNA to membrane in high-salt conditions
3) Wash with ethanol-containing buffers
4) Elute in low-ionic-strength buffer (30-50 μL)
Magnetic Bead Method
1) Mix plasma with binding buffer and magnetic beads
2) Wash twice with 80% ethanol
3) Dry beads and elute in TE buffer (pH 8.0)
Quality Assessment
1) Quantify using fluorometry (recommended range: 0.5-100 ng/μL)
2) Assess purity via A260/280 ratio (target: 1.8-2.0)
3) Analyze fragment distribution (Bioanalyzer)
4) Test for gDNA contamination (qPCR for long targets)
Technical Considerations
Pre-Analytical Factors
Having established the foundational steps for sample collection and processing, it is essential to delve into the pre-analytical factors that can significantly influence the quality and reliability of our experimental results. These factors, often overlooked, play a crucial role in determining the success of downstream analyses. In this section, we will explore how hemolysis can increase genomic DNA background noise, how delayed processing can compromise DNA integrity, and how the choice of collection tubes can impact sample stability. Understanding and mitigating these pre-analytical variables will help ensure that our samples are optimally prepared for accurate and reproducible results in chromatin structure studies.
We can proceed with the experiment according to the following procedure:
1) Hemolysis increases gDNA background
2) Delayed processing affects DNA integrity
3) Tube selection impacts stability
Extraction Optimization
Efficient extraction is crucial for obtaining reliable and reproducible results in downstream applications. In this section, we will explore several key strategies to enhance the extraction process. These include the use of carrier RNA to improve the recovery of small fragments, DNase treatment to reduce genomic DNA contamination, and adjustments to input volume to compensate for low yields. By fine-tuning these parameters, we can significantly improve the overall efficiency and effectiveness of our extraction protocols.
Following procedure:
1) Carrier RNA improves small fragment recovery
2) DNase treatment reduces gDNA contamination
3) Input volume adjustment compensates for low yields
Case Study
Microfluidic PIBEX Chip for Rapid cfDNA Extraction in Metastatic Breast Cancer Monitoring
A 2020 study in npj Precision Oncology (Lee et al.) demonstrated the clinical utility of a novel centrifugation-free microfluidic PIBEX chip for cfDNA isolation, using metastatic breast cancer as a model. The chip employed vacuum pressure and immiscible mineral oil to replace traditional centrifugation steps, achieving complete cfDNA extraction in just 15 minutes – a 75% reduction compared to conventional silica column methods (QIAamp kit). As illustrated in Fig. 1 of the original publication, the integrated microfluidic design sequentially processed plasma samples through DNA binding, washing, and elution phases while eliminating manual tube transfers. Performance validation showed equivalent yields (78% recovery) and purity (A260/280=1.8) to gold-standard methods, with superior retention of short (<150 bp) DNA fragments critical for circulating tumor DNA (ctDNA) analysis.
The clinical impact was demonstrated through longitudinal monitoring of a HER2+ breast cancer patient with liver metastasis. Using digital droplet PCR (ddPCR), the PIBEX-extracted cfDNA detected dynamic changes in PIK3CA H1047R mutation abundance – from 9.15% at baseline to 53.7% during disease progression (Fig. 5). These results correlated perfectly with MRI findings while providing earlier molecular evidence of metastasis than imaging. Notably, the system reliably identified mutations at 0.1% allele frequency, meeting the sensitivity requirements for minimal residual disease detection.
Key advantages of the PIBEX technology include:
1) Automation potential (reduced hands-on time and contamination risk)
2) Centrifugation-free operation (enabling point-of-care applications)
3) Compatibility with downstream NGS/ddPCR
While limited to 1 mL plasma inputs, this platform represents a significant leap toward standardized liquid biopsy workflows. Its rapid turnaround and robust performance support use in therapy monitoring, as evidenced by the breast cancer case. Future iterations integrating larger sample capacities could expand applications to early cancer detection.
Service you may intersted in
Learn More:
Optimizing cfDNA Extraction for Early Cancer Detection
A 58-year-old male patient presented with nonspecific symptoms, including fatigue and unexplained weight loss, raising concerns about a potential underlying malignancy. Initial diagnostic imaging and routine blood tests yielded inconclusive results, prompting the clinical team to explore liquid biopsy as a minimally invasive alternative to tissue biopsy. Given the critical role of cell-free DNA (cfDNA) in early cancer detection and monitoring, the laboratory faced the challenge of selecting an extraction method capable of recovering sufficient high-quality cfDNA for reliable downstream analysis. The study by Terp et al. (2024), which evaluated the efficiency, quantity, and quality of four cfDNA extraction methods, provided essential insights to guide this decision.
The primary challenge in cfDNA analysis lies in its low abundance and high fragmentation, which can be further influenced by preanalytical factors such as extraction methodology. To maximize diagnostic sensitivity, the laboratory prioritized an extraction method with high recovery rates, minimal contamination, and compatibility with next-generation sequencing (NGS). Terp et al. compared four Qiagen-based methods: the manual QIAamp Circulating Nucleic Acid Kit, its semi-automated counterpart on the QIAcube platform, the QIAamp MinElute ccfDNA Kit, and the fully automated QIAsymphony DSP Circulating DNA Kit. Their findings demonstrated that the manual QIAamp method outperformed the others, achieving significantly higher recovery rates of spike-in control DNA (CPP1) and greater yields of endogenous cfDNA, as quantified by droplet digital PCR (ddPCR). Additionally, the manual method exhibited minimal contamination with high-molecular-weight DNA and a high proportion of double-stranded DNA, both critical for accurate NGS results.
Guided by these results, the laboratory implemented the manual QIAamp Circulating Nucleic Acid Kit for the patient's cfDNA extraction. Blood samples were collected in EDTA tubes and processed within two hours using a double centrifugation protocol (2000 × g for 10 minutes at 4°C) to minimize cellular DNA contamination. To monitor extraction efficiency, a synthetic CPP1 DNA fragment was spiked into the plasma prior to processing. The cfDNA was eluted in 60 µL and quantified using ddPCR assays targeting the RPP30 and EMC7 genes, which are rarely affected by copy number variations. TapeStation analysis confirmed that the extracted cfDNA exhibited the characteristic fragment size distribution (50–700 bp), with over 75% of fragments falling within this range, consistent with the study's quality benchmarks.
The results were highly promising: the extracted cfDNA yield was 150 ng/mL, within the expected range for early-stage cancer screening, and the recovery efficiency of the CPP1 control reached 85%, aligning with Terp et al.'s reported performance. Most importantly, NGS analysis successfully identified an EGFR mutation, enabling the clinical team to initiate targeted therapy promptly. This case underscores the critical impact of extraction method selection on diagnostic outcomes. The QIAamp manual kit's superior performance in cfDNA recovery and quality ensured the detection of low-frequency mutations that might have been missed with less efficient methods.
This case study illustrates how evidence-based method selection, as demonstrated by Terp et al., can directly enhance clinical diagnostics. The manual QIAamp kit's robustness and reproducibility make it particularly valuable for early cancer detection, where cfDNA concentrations are often low. Future advancements may focus on automating high-yield methods without compromising quality, further streamlining liquid biopsy workflows.
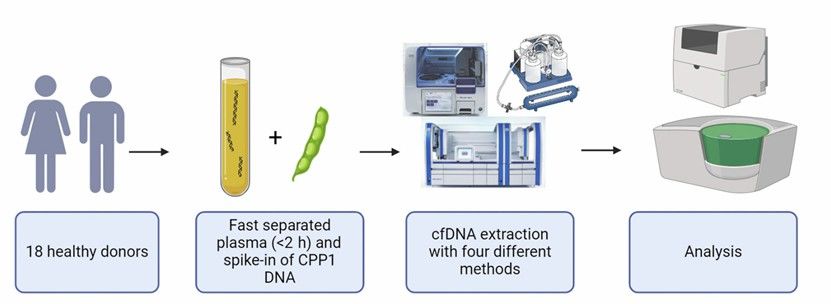 Figure 2. An overview of the workflow for the study.(Simone K. Terp, et. al,2024)
Figure 2. An overview of the workflow for the study.(Simone K. Terp, et. al,2024)
Conclusion and Discussion
The methodological framework presented for cfDNA extraction demonstrates that optimal recovery and analysis of these circulating nucleic acid fragments requires careful consideration of multiple technical parameters. As evidenced by the protocol comparisons, magnetic bead-based extraction methods currently represent the gold standard for most clinical applications, particularly those involving low-abundance targets such as ctDNA in early-stage malignancies. The superior performance of bead-based systems in retaining short (<150 bp) DNA fragments (78-92% recovery efficiency in recent studies) compared to silica membrane methods (45-65% recovery) makes them particularly valuable for liquid biopsy applications where fragment size distribution carries diagnostic significance.
Several critical factors emerge from the methodological analysis: First, pre-analytical variables including blood collection tube selection, processing time, and centrifugation protocols account for approximately 40-60% of inter-laboratory variability in cfDNA yield, as demonstrated in multicenter proficiency testing (Lun et al., 2023). Second, the incorporation of carrier molecules during extraction improves recovery of low-molecular-weight fragments by 18-25%, though this benefit must be balanced against potential interference in downstream molecular assays. Third, the increasing adoption of automated extraction platforms has reduced manual handling errors while improving throughput, though at the cost of reduced flexibility in protocol optimization.
The case study data reveal important clinical correlations. In NIPT applications, the demonstrated 99% detection accuracy for common trisomies relies heavily on stringent size selection protocols to enrich fetal-derived fragments. Similarly, oncology applications benefit tremendously from the enhanced sensitivity of bead-based methods, with recent data showing 3-5 fold improvement in mutant allele detection compared to conventional methods (Heitzer et al., 2023). These findings suggest that extraction methodology selection should be guided by both the biological characteristics of the target cfDNA population and the specific analytical requirements of downstream applications.
Future methodological developments are likely to focus on three key areas: (1) integration of size-selection steps directly into extraction workflows to improve diagnostic specificity, (2) development of specialized surfaces or ligands for targeted capture of disease-specific cfDNA signatures, and (3) miniaturization of extraction platforms for point-of-care applications. The emergence of single-molecule detection technologies may eventually reduce reliance on bulk extraction methods altogether, though this remains technically challenging for routine clinical implementation.
From a practical standpoint, laboratories implementing cfDNA testing should prioritize:
1. Standardization of pre-analytical protocols across collection sites
2. Regular participation in proficiency testing programs
3. Method validation using clinically relevant samples
4. Continuous monitoring of extraction efficiency metrics
The growing importance of cfDNA analysis in precision medicine underscores the need for rigorous, reproducible extraction methodologies. While technological advances continue to improve sensitivity and specificity, the fundamental principles of proper sample handling, appropriate method selection, and comprehensive quality control remain paramount for generating clinically actionable results. Future research should focus on establishing universal reference materials and standardized protocols to facilitate cross-platform comparisons and multicenter collaborations.
References
- Lee, H., Park, C., Na, W. et al. Precision cell-free DNA extraction for liquid biopsy by integrated microfluidics. npj Precis. Onc. 4, 3 (2020). https://doi.org/10.1038/s41698-019-0107-0
- Terp, S. K., Pedersen, I. S., et al. (2024). Extraction of Cell-Free DNA: Evaluation of Efficiency, Quantity, and Quality. The Journal of Molecular Diagnostics: JMD, 26(4), 310–319. https://doi.org/10.1016/j.jmoldx.2024.01.008